Abstract
Human O-GlcNAcase (hOGA) is the unique enzyme responsible for the hydrolysis of the O-linked β-N-acetyl glucosamine (O-GlcNAc) modification, an essential protein glycosylation event that modulates the function of numerous cellular proteins in response to nutrients and stress. Here we report crystal structures of a truncated hOGA, which comprises the catalytic and stalk domains, in apo form, in complex with an inhibitor, and in complex with a glycopeptide substrate. We found that hOGA forms an unusual arm-in-arm homodimer in which the catalytic domain of one monomer is covered by the stalk domain of the sister monomer to create a substrate-binding cleft. Notably, the residues on the cleft surface afford extensive interactions with the peptide substrate in a recognition mode that is distinct from that of its bacterial homologs. These structures represent the first model of eukaryotic enzymes in the glycoside hydrolase 84 (GH84) family and provide a crucial starting point for understanding the substrate specificity of hOGA, which regulates a broad range of biological and pathological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Torres, C.R. & Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 (1984).
Hanover, J.A., Krause, M.W. & Love, D.C. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 13, 312–321 (2012).
Ma, J. & Hart, G.W. O-GlcNAc profiling: from proteins to proteomes. Clin. Proteomics 11, 8 (2014).
Bond, M.R. & Hanover, J.A. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880 (2015).
Hardivillé, S. & Hart, G.W. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 20, 208–213 (2014).
Lewis, B.A. & Hanover, J.A. O-GlcNAc and the epigenetic regulation of gene expression. J. Biol. Chem. 289, 34440–34448 (2014).
Lewis, B.A. O-GlcNAcylation at promoters, nutrient sensors, and transcriptional regulation. Biochim. Biophys. Acta 1829, 1202–1206 (2013).
Lazarus, M.B. et al. HCF-1 is cleaved in the active site of O-GlcNAc transferase. Science 342, 1235–1239 (2013).
Drougat, L. et al. Characterization of O-GlcNAc cycling and proteomic identification of differentially O-GlcNAcylated proteins during G1/S transition. Biochim. Biophys. Acta 1820, 1839–1848 (2012).
Zachara, N.E. & Hart, G.W. O-GlcNAc modification: a nutritional sensor that modulates proteasome function. Trends Cell Biol. 14, 218–221 (2004).
Guinez, C. et al. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 22, 2901–2911 (2008).
Vaidyanathan, K. & Wells, L. Multiple tissue-specific roles for the O-GlcNAc post-translational modification in the induction of, and complications arising from, type 2 diabetes. J. Biol. Chem. 289, 34466–34471 (2014).
Ma, J. & Hart, G.W. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev. Proteomics 10, 365–380 (2013).
Zhu, Y., Shan, X., Yuzwa, S.A. & Vocadlo, D.J. The emerging link between O-GlcNAc and Alzheimer's disease. J. Biol. Chem. 289, 34472–34481 (2014).
Yuzwa, S.A. & Vocadlo, D.J. O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer's disease and beyond. Chem. Soc. Rev. 43, 6839–6858 (2014).
Ferrer, C.M., Sodi, V.L. & Reginato, M.J. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J. Mol. Biol. 428, 3282–3294 (2016).
Ma, Z. & Vosseller, K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J. Biol. Chem. 289, 34457–34465 (2014).
Singh, J.P., Zhang, K., Wu, J. & Yang, X. O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett. 356, 2 Pt A, 244–250 (2015).
Kreppel, L.K., Blomberg, M.A. & Hart, G.W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 (1997).
Gao, Y., Wells, L., Comer, F.I., Parker, G.J. & Hart, G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 (2001).
Pathak, S. et al. The active site of O-GlcNAc transferase imposes constraints on substrate sequence. Nat. Struct. Mol. Biol. 22, 744–750 (2015).
Lazarus, M.B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469, 564–567 (2011).
Lazarus, M.B. et al. Structural snapshots of the reaction coordinate for O-GlcNAc transferase. Nat. Chem. Biol. 8, 966–968 (2012).
Schimpl, M., Schüttelkopf, A.W., Borodkin, V.S. & van Aalten, D.M.F. Human OGA binds substrates in a conserved peptide-recognition groove. Biochem. J. 432, 1–7 (2010).
Vocadlo, D.J. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr. Opin. Chem. Biol. 16, 488–497 (2012).
Alonso, J., Schimpl, M. & van Aalten, D.M.F. O-GlcNAcase: promiscuous hexosaminidase or key regulator of O-GlcNAc signaling? J. Biol. Chem. 289, 34433–34439 (2014).
Rao, F.V. et al. Structure of a bacterial putative acetyltransferase defines the fold of the human O-GlcNAcase C-terminal domain. Open Biol. 3, 130021 (2013).
He, Y., Roth, C., Turkenburg, J.P. & Davies, G.J. Three-dimensional structure of a Streptomyces sviceus GNAT acetyltransferase with similarity to the C-terminal domain of the human GH84 O-GlcNAcase. Acta Crystallogr. D Biol. Crystallogr. 70, 186–195 (2014).
Toleman, C., Paterson, A.J., Whisenhunt, T.R. & Kudlow, J.E. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activatable O-GlcNAcase and HAT activities. J. Biol. Chem. 279, 53665–53673 (2004).
Keembiyehetty, C.N., Krzes´lak, A., Love, D.C. & Hanover, J.A. A lipid-droplet-targeted O-GlcNAcase isoform is a key regulator of the proteasome. J. Cell Sci. 124, 2851–2860 (2011).
Kim, E.J., Kang, D.O., Love, D.C. & Hanover, J.A. Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohydr. Res. 341, 971–982 (2006).
Li, J. et al. Isoforms of human O-GlcNAcase show distinct catalytic efficiencies. Biochemistry 75, 938–943 (2010).
Macauley, M.S., Whitworth, G.E., Debowski, A.W., Chin, D. & Vocadlo, D.J. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 280, 25313–25322 (2005).
Whitworth, G.E. et al. Analysis of PUGNAc and NAG-thiazoline as transition-state analogs for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition-state poise. J. Am. Chem. Soc. 129, 635–644 (2007).
He, Y., Bubb, A.K., Stubbs, K.A., Gloster, T.M. & Davies, G.J. Inhibition of a bacterial O-GlcNAcase homolog by lactone and lactam derivatives: structural, kinetic, and thermodynamic analyses. Amino Acids 40, 829–839 (2011).
Dennis, R.J. et al. Structure and mechanism of a bacterial β-glucosaminidase having O-GlcNAcase activity. Nat. Struct. Mol. Biol. 13, 365–371 (2006).
Yuzwa, S.A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 (2008).
He, Y., Macauley, M.S., Stubbs, K.A., Vocadlo, D.J. & Davies, G.J. Visualizing the reaction coordinate of an O-GlcNAc hydrolase. J. Am. Chem. Soc. 132, 1807–1809 (2010).
Rao, F.V. et al. Structural insights into the mechanism and inhibition of eukaryotic O-GlcNAc hydrolysis. EMBO J. 25, 1569–1578 (2006).
Schimpl, M., Borodkin, V.S., Gray, L.J. & van Aalten, D.M.F. Synergy of peptide and sugar in O-GlcNAcase substrate recognition. Chem. Biol. 19, 173–178 (2012).
Ostrowski, A., Gundogdu, M., Ferenbach, A.T., Lebedev, A.A. & van Aalten, D.M.F. Evidence for a functional O-linked N-acetylglucosamine (O-GlcNAc) system in the thermophilic bacterium Thermobaculum terrenum. J. Biol. Chem. 290, 30291–30305 (2015).
Macauley, M.S. & Vocadlo, D.J. Increasing O-GlcNAc levels: an overview of small-molecule inhibitors of O-GlcNAcase. Biochim. Biophys. Acta 1800, 107–121 (2010).
Banerjee, P.S., Hart, G.W. & Cho, J.W. Chemical approaches to study O-GlcNAcylation. Chem. Soc. Rev. 42, 4345–4357 (2013).
Dorfmueller, H.C. et al. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-GlcNAcylation levels. J. Am. Chem. Soc. 128, 16484–16485 (2006).
Cetinbaş, N., Macauley, M.S., Stubbs, K.A., Drapala, R. & Vocadlo, D.J. Identification of Asp174 and Asp175 as the key catalytic residues of human O-GlcNAcase by functional analysis of site-directed mutants. Biochemistry 45, 3835–3844 (2006).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Yuzwa, S.A. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 8, 393–399 (2012).
Yu, Y. et al. Differential effects of an O-GlcNAcase inhibitor on tau phosphorylation. PLoS One 7, e35277 (2012).
Lameira, J. et al. Quantum mechanical–molecular mechanical molecular dynamics simulation of wild-type and seven mutants of CpNagJ in complex with PUGNAc. J. Phys. Chem. B 114, 7029–7036 (2010).
Shen, D.L., Gloster, T.M., Yuzwa, S.A. & Vocadlo, D.J. Insights into O-linked N-acetylglucosamine ([0–9]O-GlcNAc) processing and dynamics through kinetic analysis of O-GlcNAc transferase and O-GlcNAcase activity on protein substrates. J. Biol. Chem. 287, 15395–15408 (2012).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
DeLano, W.L. The PyMOL Molecular Graphics System (Delano Scientific LLC, 2002).
Laue, T.M. Sedimentation equilibrium as a thermodynamic tool. Methods Enzymol. 259, 427–452 (1995).
Xie, D., Suvorov, L., Erickson, J.W. & Gulnik, A.S. Real-time measurements of dark-substrate catalysis. Protein Sci. 8, 2460–2464 (1999).
Acknowledgements
We thank D. Smith and L. Carlson for assistance with X-ray data collection, K. Satyshur and D. McCaslin for helpful discussions, and members of the Jiang laboratory for critical reading of the manuscript. This work was supported by start-up funds from the University of Wisconsin–Madison (J.J.).
Author information
Authors and Affiliations
Contributions
J.J. oversaw all aspects of the experiments and manuscript preparation; B.L. performed cloning, mutagenesis, protein purification, enzymatic assays, analytical ultracentrifugation sedimentation equilibrium experiments, crystallization, and structural determination; H.L. synthesized thiamet-G; L.L. assisted with molecular cloning and protein purification; and B.L. and J.J. wrote the manuscript. All coauthors participated in editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Substrate-assisted mechanism of OGA.
OGA-catalyzed hydrolysis of O-GlcNAcylation via a substrate-assisted mechanism, adapted from a previous report (Dennis, R. J. et al., Nat. Struct. Mol. Biol. 13, 365–371, 2006). The roles of ancillary residues Lys98 and Tyr219 are inferred from the crystal structure of OGAcryst–thiamet-G from this study.
Supplementary Figure 2 Kinetic parameters of hOGA and OGAcryst proteins.
(a) Michaelis–Menten plots of hOGA and OGAcryst with varying concentrations of 4MU-NAG. Data were fitted using GraphPad Prism. Error bars represent s.d. values derived from three independent experiments. (b) Summary of the kinetic parameters of hOGA and OGAcryst. The Km and kcat were determined from three independent experiments and displayed as average ± s.d.
Supplementary Figure 3 Structural comparison of the two sister monomers of OGAcryst.
Two perpendicular views of superimposed OGAα and OGAβ reveal that the catalytic domains are folded into an identical (β/α)8-barrel, while the stalk domains display a slight variation. OGAα is colored yellow; the catalytic domain and stalk domain of OGAβ are colored magenta and cyan, respectively.
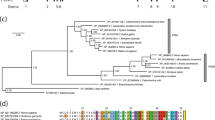
Supplementary Figure 4 The sequence and structure of the hOGA stalk domain are markedly different from those of its bacterial homologs.
(a) Sequence alignment and (b-c) structural comparison of the stalk domains of OGA proteins from human (hOGA, cyan) and representative bacterial species: Oceanicola granulosus (OgOGA, PDB: 2XSA, magenta) (Schimpl, M. et al., Biochem. J. 432, 1–7, 2010), Bacteroides thetaiotaomicron (BtGH84, PDB: 2CHO, orange) (Dennis, R. J. et al., Nat. Struct. Mol. Biol. 13, 365–371, 2006), Clostridium perfringens (CpOGA, PDB: 2CBJ, green) (Rao, F. V. et al., EMBO J. 25, 1569–1578, 2006) and Thermobaculum terrenum (TtOGA, PDB: 5DIY, grey) (Ostrowski, A., et al., J. Biol. Chem. 290, 30291–30305, 2015). In the sequence alignment, variable and similar residues are shown in black and red, respectively.
Supplementary Figure 5 Extensive polar interactions stabilize the dimerization of OGAcryst.
(a) Two different views of OGAcryst with dimerization interface. OGAα is colored gray; the catalytic domain and stalk domain of OGAβ are colored magenta and cyan, respectively. (b) Schematic representation of the hydrogen bonds detected at the dimerization interface. (c) Schematic representation of the salt bridges detected at the dimerization interface. Residues from OGAα and OGAβ participating in the polar interactions are colored in yellow and green, respectively.
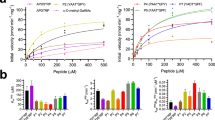
Supplementary Figure 6 Relative activities and substrate-binding evaluation of hOGA variants.
(a) The activities of hOGA mutants were measured using 4MU-NAG and normalized to the wild-type enzyme. The error bars represent the s.d. values derived from three independent experiments. (b) To evaluate the contribution of hydrophobic interactions with the p53 glycopeptide (Ac-QLWVDS(O-GlcNAc)TPPPG), the Michaelis constants of hOGA and mutants were determined using 4MU-NAG as the reporter substrate following multisubstrate enzyme kinetics as shown previously (Schimpl, M. et al., Biochem. J. 432, 1–7, 2010; Schimpl, M. et al., Chem. Biol. 19, 173–178, 2012; Xie, D. et al., Protein Science 8, 2460–2464, 1999). *The Michaelis constant of wild-type hOGA was measured towards a W(-3)A mutant of p53 glycopeptide (Ac-QLAVDS(O-GlcNAc)TPPPG) using a similar assay. The Km´ values were determined from three independent experiments and displayed as average ± s.d.
Supplementary Figure 7 Binding conformation of p53 glycopeptides in the OGAcryst–p53 complex.
(a) Fo–Fc difference map of p53 glycopeptides from the OGAcryst–p53 complex (contoured at 3σ). The peptides from OGAα and OGAβ are shown in orange and green sticks, respectively. Intramolecular hydrogen bonds are displayed as dotted lines. (b) Superposition of OGAcryst–p53 complex structures collected from three independent soaking experiments (inserts: enlarged p53 glycopeptides from each monomer. The p53 glycopeptides in the right insert have been rotated for better clarity). (c-d) Close-up views of the binding conformations of p53 glycopeptides in (c) OGAα and (d) OGAβ. Residues on the inner surface of the substrate-binding cleft that are in close vicinity of the peptide are highlighted in (c) blue sticks and (d) yellow sticks, respectively. Hydrogen bonds are displayed in dashed lines. (e-f) Crystal packing potentially contributes to stabilize p53 glycopeptide in OGAβ but not in OGAα. OGAα and OGAβ are shown in magenta and yellow, respectively. The symmetric molecule is shown in grey.
Supplementary Figure 8 The p53 glycopeptide adopts distinct binding conformations in the structures of human and bacterial OGAs.
(a) Superposition of the p53 glycopeptide (stick representation) in the CpOGA–p53 complex (PDB: 2YDR) (Schimpl, M. et al., Chem. Biol. 19, 173–178, 2012) with those in the OGAcryst–p53 complex. (b) Surface representation of the CpOGA–p53 complex, highlighting the p53 glycopeptide (yellow) bound on top of the active site (PDB: 2YDR). (c) Surface representation of the p53 glycopeptide (orange) bound in the substrate-binding cleft that comprises the catalytic domain of OGAα (grey) and the stalk domain of OGAβ (cyan). (d) Surface representation of the p53 glycopeptide (green) bound in the substrate-binding cleft that comprises the stalk domain of OGAα (grey) and the catalytic domain of OGAβ (magenta). In b-d panels, stick presentation of p53 glycopeptides is displayed as semitransparent.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–2 and Supplementary Note 1 (PDF 8701 kb)
Rights and permissions
About this article
Cite this article
Li, B., Li, H., Lu, L. et al. Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat Struct Mol Biol 24, 362–369 (2017). https://doi.org/10.1038/nsmb.3390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3390
This article is cited by
-
O-GlcNAc forces an α-synuclein amyloid strain with notably diminished seeding and pathology
Nature Chemical Biology (2024)
-
Cryo-EM structure of human O-GlcNAcylation enzyme pair OGT-OGA complex
Nature Communications (2023)
-
Enhanced production of N-acetyl-glucosaminidase by marine Aeromonas caviae CHZ306 in bioreactor
Brazilian Journal of Microbiology (2023)
-
Target protein deglycosylation in living cells by a nanobody-fused split O-GlcNAcase
Nature Chemical Biology (2021)
-
O-GlcNAcylation: the “stress and nutrition receptor” in cell stress response
Cell Stress and Chaperones (2021)