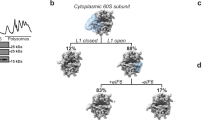
Abstract
SBDS protein (deficient in the inherited leukemia-predisposition disorder Shwachman-Diamond syndrome) and the GTPase EFL1 (an EF-G homolog) activate nascent 60S ribosomal subunits for translation by catalyzing eviction of the antiassociation factor eIF6 from nascent 60S ribosomal subunits. However, the mechanism is completely unknown. Here, we present cryo-EM structures of human SBDS and SBDS–EFL1 bound to Dictyostelium discoideum 60S ribosomal subunits with and without endogenous eIF6. SBDS assesses the integrity of the peptidyl (P) site, bridging uL16 (mutated in T-cell acute lymphoblastic leukemia) with uL11 at the P-stalk base and the sarcin-ricin loop. Upon EFL1 binding, SBDS is repositioned around helix 69, thus facilitating a conformational switch in EFL1 that displaces eIF6 by competing for an overlapping binding site on the 60S ribosomal subunit. Our data reveal the conserved mechanism of eIF6 release, which is corrupted in both inherited and sporadic leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
Electron Microscopy Data Bank
Protein Data Bank
Referenced accessions
NCBI Reference Sequence
Protein Data Bank
References
Lo, K.Y. et al. Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol. Cell 39, 196–208 (2010).
Boocock, G.R. et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 33, 97–101 (2003).
Senger, B. et al. The nucle(ol)ar Tif6p and Efl1p are required for a late cytoplasmic step of ribosome synthesis. Mol. Cell 8, 1363–1373 (2001).
Menne, T.F. et al. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat. Genet. 39, 486–495 (2007).
Finch, A.J. et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes Dev. 25, 917–929 (2011).
Wong, C.C., Traynor, D., Basse, N., Kay, R.R. & Warren, A.J. Defective ribosome assembly in Shwachman-Diamond syndrome. Blood 118, 4305–4312 (2011).
Gartmann, M. et al. Mechanism of eIF6-mediated inhibition of ribosomal subunit joining. J. Biol. Chem. 285, 14848–14851 (2010).
Klinge, S., Voigts-Hoffmann, F., Leibundgut, M., Arpagaus, S. & Ban, N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334, 941–948 (2011).
Ban, N. et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 24, 165–169 (2014).
Ceci, M. et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature 426, 579–584 (2003).
Basu, U., Si, K., Warner, J.R. & Maitra, U. The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis. Mol. Cell. Biol. 21, 1453–1462 (2001).
Donadieu, J. et al. Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia: experience of the French Severe Chronic Neutropenia Study Group. Haematologica 90, 45–53 (2005).
De Keersmaecker, K. et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat. Genet. 45, 186–190 (2013).
Shammas, C. et al. Structural and mutational analysis of the SBDS protein family: insight into the leukemia-associated Shwachman-Diamond Syndrome. J. Biol. Chem. 280, 19221–19229 (2005).
Savchenko, A. et al. The Shwachman-Bodian-Diamond syndrome protein family is involved in RNA metabolism. J. Biol. Chem. 280, 19213–19220 (2005).
Ng, C.L. et al. Conformational flexibility and molecular interactions of an archaeal homologue of the Shwachman-Bodian-Diamond syndrome protein. BMC Struct. Biol. 9, 32 (2009).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334, 1524–1529 (2011).
Voorhees, R.M., Fernandez, I.S., Scheres, S.H. & Hegde, R.S. Structure of the mammalian ribosome-Sec61 complex to 3.4 A resolution. Cell 157, 1632–1643 (2014).
Khatter, H., Myasnikov, A.G., Natchiar, S.K. & Klaholz, B.P. Structure of the human 80S ribosome. Nature 520, 640–645 (2015).
Greber, B.J. et al. Cryo-EM structure of the archaeal 50S ribosomal subunit in complex with initiation factor 6 and implications for ribosome evolution. J. Mol. Biol. 418, 145–160 (2012).
Sulima, S.O. et al. Eukaryotic rpL10 drives ribosomal rotation. Nucleic Acids Res. 42, 2049–2063 (2014).
Gao, Y.G. et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326, 694–699 (2009).
Anger, A.M. et al. Structures of the human and Drosophila 80S ribosome. Nature 497, 80–85 (2013).
Graindorge, J.S. et al. Deletion of EFL1 results in heterogeneity of the 60 S GTPase-associated rRNA conformation. J. Mol. Biol. 352, 355–369 (2005).
Schmeing, T.M. et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326, 688–694 (2009).
Becker, T. et al. Structure of the no-go mRNA decay complex Dom34-Hbs1 bound to a stalled 80S ribosome. Nat. Struct. Mol. Biol. 18, 715–720 (2011).
Basu, U., Si, K., Deng, H. & Maitra, U. Phosphorylation of mammalian eukaryotic translation initiation factor 6 and its Saccharomyces cerevisiae homologue Tif6p: evidence that phosphorylation of Tif6p regulates its nucleocytoplasmic distribution and is required for yeast cell growth. Mol. Cell. Biol. 23, 6187–6199 (2003).
Ray, P. et al. The Saccharomyces cerevisiae 60 S ribosome biogenesis factor Tif6p is regulated by Hrr25p-mediated phosphorylation. J. Biol. Chem. 283, 9681–9691 (2008).
Bussiere, C., Hashem, Y., Arora, S., Frank, J. & Johnson, A.W. Integrity of the P-site is probed during maturation of the 60S ribosomal subunit. J. Cell Biol. 197, 747–759 (2012).
Hauryliuk, V., Hansson, S. & Ehrenberg, M. Cofactor dependent conformational switching of GTPases. Biophys. J. 95, 1704–1715 (2008).
Lancaster, L., Kiel, M.C., Kaji, A. & Noller, H.F. Orientation of ribosome recycling factor in the ribosome from directed hydroxyl radical probing. Cell 111, 129–140 (2002).
Wilson, D.N. et al. X-ray crystallography study on ribosome recycling: the mechanism of binding and action of RRF on the 50S ribosomal subunit. EMBO J. 24, 251–260 (2005).
Gao, N., Zavialov, A.V., Ehrenberg, M. & Frank, J. Specific interaction between EF-G and RRF and its implication for GTP-dependent ribosome splitting into subunits. J. Mol. Biol. 374, 1345–1358 (2007).
Weixlbaumer, A. et al. Crystal structure of the ribosome recycling factor bound to the ribosome. Nat. Struct. Mol. Biol. 14, 733–737 (2007).
Seshadri, A., Singh, N.S. & Varshney, U. Recycling of the posttermination complexes of Mycobacterium smegmatis and Escherichia coli ribosomes using heterologous factors. J. Mol. Biol. 401, 854–865 (2010).
Hedges, J., West, M. & Johnson, A.W. Release of the export adapter, Nmd3p, from the 60S ribosomal subunit requires Rpl10p and the cytoplasmic GTPase Lsg1p. EMBO J. 24, 567–579 (2005).
Strunk, B.S., Novak, M.N., Young, C.L. & Karbstein, K. A translation-like cycle is a quality control checkpoint for maturing 40S ribosome subunits. Cell 150, 111–121 (2012).
Lebaron, S. et al. Proofreading of pre-40S ribosome maturation by a translation initiation factor and 60S subunits. Nat. Struct. Mol. Biol. 19, 744–753 (2012).
Sengupta, J. et al. Characterization of the nuclear export adaptor protein Nmd3 in association with the 60S ribosomal subunit. J. Cell Biol. 189, 1079–1086 (2010).
Greber, B.J., Boehringer, D., Montellese, C. & Ban, N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat. Struct. Mol. Biol. 19, 1228–1233 (2012).
Bradatsch, B. et al. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. Nat. Struct. Mol. Biol. 19, 1234–1241 (2012).
Valli, R. et al. Different loss of material in recurrent chromosome 20 interstitial deletions in Shwachman-Diamond syndrome and in myeloid neoplasms. Mol. Cytogenet. 6, 56 (2013).
Yokoyama, T. et al. Structural insights into initial and intermediate steps of the ribosome-recycling process. EMBO J. 31, 1836–1846 (2012).
van Heel, M., Harauz, G., Orlova, E.V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996).
Bai, X.C., Fernandez, I.S., McMullan, G. & Scheres, S.H. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife 2, e00461 (2013).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Scheres, S.H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012).
Scheres, S.H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Klaholz, B.P., Myasnikov, A.G. & Van Heel, M. Visualization of release factor 3 on the ribosome during termination of protein synthesis. Nature 427, 862–865 (2004).
Penczek, P.A., Frank, J. & Spahn, C.M. A method of focused classification, based on the bootstrap 3D variance analysis, and its application to EF-G-dependent translocation. J. Struct. Biol. 154, 184–194 (2006).
Scheres, S.H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Rosenthal, P.B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Larkin, M.A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Jossinet, F., Ludwig, T.E. & Westhof, E. Assemble: an interactive graphical tool to analyze and build RNA architectures at the 2D and 3D levels. Bioinformatics 26, 2057–2059 (2010).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Amunts, A. et al. Structure of the yeast mitochondrial large ribosomal subunit. Science 343, 1485–1489 (2014).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 (1996).
Trabuco, L.G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Mackerell, A.D. Jr., Feig, M. & Brooks, C.L. III. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 25, 1400–1415 (2004).
MacKerell, A.D. Jr., Banavali, N. & Foloppe, N. Development and current status of the CHARMM force field for nucleic acids. Biopolymers 56, 257–265 (2000).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Acknowledgements
We thank A. Johnson (University of Texas) and A. Newman (Medical Research Council Laboratory of Molecular Biology) for yeast strains; B. Trumpower (Dartmouth Medical School) for providing uL16 antiserum; S. Chen, C. Savva, S. De Carlo, S. Welsch, F. De Haas, M. Vos and K. Sader for technical support with cryo-EM; G. McMullan for help with movie data acquisition; T. Darling and J. Grimmett for help with computing; A. Brown for help with refinement; and S. Scheres for discussion. This work was supported by a Federation of European Biochemical Societies Long Term Fellowship (to F.W.), the Specialist Programme from Bloodwise (12048 to A.J.W.), the UK Medical Research Council (MRC) (MC_U105161083 to A.J.W. and U105115237 to R.R.K.), a Wellcome Trust strategic award to the Cambridge Institute for Medical Research (100140), a core support grant from the Wellcome Trust and MRC to the Wellcome Trust–Medical Research Council Cambridge Stem Cell Institute, the Tesni Parry Trust (to A.J.W.), Ted's Gang (to A.J.W.) and the Cambridge National Institute for Health Research Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
F.W. performed sample preparation, EM data collection, image processing and model refinement. E.G. performed model building and fitting; M.C., L.J. and A.J.W. performed genetic and biochemical experiments; C.H. performed protein expression and purification; C.C.W. generated mutant Dictyostelium strains with advice from D.T. and R.R.K.; and D.T. and F.W. cultured Dictyostelium cells. F.W. and A.J.W. designed experiments and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Overview of cryo-EM image-processing procedure.
Maximum likelihood classification scheme and masks used to obtain the complexes of eIF6, SBDS and EFL1 bound to the 60S ribosomal subunit. Insets show the position of the spherical masks (grey) on the 60S subunit (cyan).
Supplementary Figure 2 Resolution of the 60S-ribosomal-subunit complexes and validation of the atomic model.
(a) Gold-standard Fourier shell correlation (FSC) curves for the three 60S ribosome complexes after 3D refinement and statistical movie processing in RELION (Bai, X. C. et al., Elife 2, e00461, 2013; Scheres, S. H. J Struct Biol 180, 519-530, 2012).
(b) Surface (top panel) and cross-sectional (bottom panel) views of the unsharpened final maps colored according to local resolution calculated with the ResMap software package (Kucukelbir, A. et al., Nat Methods, 11, 63-65, 2014).
(c, d, e, f) Examples of densities with the model fitted showing helix 72 of the 26S rRNA from the 60S-eIF6-SBDS complex with the density filtered at 3.3 Å (c), α-helix of the uL6 protein from the 60S-eIF6-SBDS complex with the density filtered at 3.3 Å (d), SBDS from the 60S-eIF6-SBDS complex with the density is filtered at 4 Å (e), EFL1 from the 60S-eIF6-SBDS-EFL1 complex with the density is filtered at 8 Å (f).
(g, h, i) Cross-validation against over-fitting. FSC curves are shown for the 60S-eIF6-SBDS (g), 60S-eIF6-SBDS-EFL1 (h) and 60S-SBDS-EFL1 (i) complexes between the final refined atomic model and the reconstructions from all particles (black); between the model refined in the reconstruction from only half of the particles and the reconstruction from that same half (FSCwork, red); and between that same model and the reconstruction from the other half of the particles (FSCtest, green).
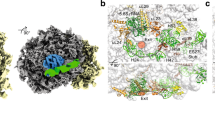
Supplementary Figure 3 Atomic models of the 60S–SBDS–eIF6 complex.
(a) Secondary structure diagram of modeled Dictyostelium 26S rRNA. The diagram was modified from S. cerevisiae 25S rRNA (Comparative RNA Web Site, www.rna.ccbb.utexas.edu). The PTC is shown in bold. Base pairs involved in tertiary interactions are indicated with lines connecting the circles or boxes around the bases involved in the interaction. (-) corresponds to canonical base pairs, while (•, ○ and ●) correspond to non-canonical base pairs.
(b, c) Back (b) and front (c) views of the atomic model of the 60S-eIF6-SBDS complex. SRL, sarcin-ricin loop.
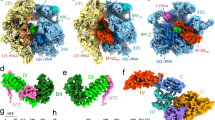
Supplementary Figure 4 Molecular environment of eIF6, SBDS and EFL1.
(a, b) Schematic representations of Homo sapiens EF-2 (a) and EFL1 (b).
(c) Ribbon representation of the atomic model of human EFL1 with the subdomain structure highlighted using the same color scheme as (b).
(d-g) Back (d, f) and front (e, g) views of the atomic models of the 60S-eIF6-SBDS-EFL1 and 60S-SBDS-EFL1 complexes, respectively. SRL, sarcin-ricin loop.
Supplementary Figure 5 uL16 is required to recruit Sdo1 to the 60S subunit in vivo.
(a) The EFL1 domain II insertion is dispensable in vivo. Random sporulation assay showing efl1Δ cells transformed with empty vector (pRS316) control or plasmids expressing wild type EFL1 or the EFL1Δ420-580 mutant (left).
(b) Tenfold serial dilutions (left to right) of the indicated yeast cells spotted onto selective –URA medium (left). efl1Δ cells transformed with empty vector (pRS316) are non-viable and are therefore not shown.
(c) S174 and S175 are dispensable for Tif6 function in vivo. Random sporulation assay showing tif6Δ cells transformed with empty vector (pRS316) control or plasmids expressing wild type TIF6 or the indicated TIF6 mutants.
(d) Tenfold serial dilutions (left to right) of the indicated yeast strains spotted onto selective –URA medium (left). tif6Δ cells transformed with empty vector (pRS316) are non-viable and are therefore not shown.
(e) Severe fitness defect of the uL16-H123P allele. Indicated yeast cells with (galactose) or without (glucose) endogenous uL16 spotted in tenfold serial dilutions (left to right) onto solid media.
(f) The uL16-H123P allele markedly reduces uL16 expression. uL16 and uL14 (control) were visualized by immunoblotting of extracts from the indicated strains with (galactose) or without (glucose) endogenous uL16. As controls, uL16 and uL14 were visualized by immunoblotting of extracts from strain C375 (wild type).
(g) The T-ALL associated alleles uL16-H123P, uL16-R98S and uL16-R98C impair Sdo1 binding to the 60S subunit in vivo. Sdo1-FLAG or uL14 were visualized by immunoblotting of supernatant (S) or pellet (P) from cell extracts with (galactose) or without (glucose) endogenous uL16 across the indicated range of NaCl concentrations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–6 (PDF 1810 kb)
Supplementary Data Set 1
Uncropped blots related to Fig. 4 (PDF 16051 kb)
Rights and permissions
About this article
Cite this article
Weis, F., Giudice, E., Churcher, M. et al. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat Struct Mol Biol 22, 914–919 (2015). https://doi.org/10.1038/nsmb.3112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3112
This article is cited by
-
Convergent somatic evolution commences in utero in a germline ribosomopathy
Nature Communications (2023)
-
RSL24D1 sustains steady-state ribosome biogenesis and pluripotency translational programs in embryonic stem cells
Nature Communications (2023)
-
eIF6 is potential diagnostic and prognostic biomarker that associated with 18F-FDG PET/CT features and immune signatures in esophageal carcinoma
Journal of Translational Medicine (2022)
-
Shwachman Diamond syndrome: narrow genotypic spectrum and variable clinical features
Pediatric Research (2022)
-
Fractional 2′-O-methylation in the ribosomal RNA of Dictyostelium discoideum supports ribosome heterogeneity in Amoebozoa
Scientific Reports (2022)