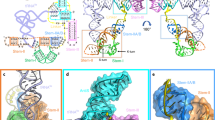
Abstract
The bacterial alarmone 5-aminoimidazole-4-carboxamide riboside 5′-triphosphate (AICAR triphosphate or ZTP), derived from the monophosphorylated purine precursor ZMP, accumulates during folate starvation. ZTP regulates genes involved in purine and folate metabolism through a cognate riboswitch. The linker connecting this riboswitch's two subdomains varies in length by over 100 nucleotides. We report the cocrystal structure of the Fusobacterium ulcerans riboswitch bound to ZMP, which spans the two subdomains whose interface also comprises a pseudoknot and ribose zipper. The riboswitch recognizes the carboxamide oxygen of ZMP through an unprecedented inner-sphere coordination with a Mg2+ ion. We show that the affinity of the riboswitch for ZMP is modulated by the linker length. Notably, ZMP can simultaneously bind to the two subdomains even when they are synthesized as separate RNAs. The ZTP riboswitch demonstrates how specific small-molecule binding can drive association of distant noncoding-RNA domains to regulate gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Referenced accessions
NCBI Reference Sequence
References
Hartman, S.C. & Buchanan, J.M. Nucleic acids, purines, pyrimidines (nucleotide synthesis). Annu. Rev. Biochem. 28, 365–410 (1959).
Bochner, B.R. & Ames, B.N. ZTP (5-amino 4-imidazole carboxamide riboside 5′-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell 29, 929–937 (1982).
Kim, P.B., Nelson, J.W. & Breaker, R.R. An ancient riboswitch class in bacteria regulates purine biosynthesis and one-carbon metabolism. Mol. Cell 57, 317–328 (2015).
Peselis, A. & Serganov, A. Themes and variations in riboswitch structure and function. Biochim. Biophys. Acta 1839, 908–918 (2014).
Jones, C.P. & Ferré-D'Amaré, A.R. RNA quaternary structure and global symmetry. Trends Biochem. Sci. 40, 211–220 (2015).
Batey, R.T. Structure and mechanism of purine-binding riboswitches. Q. Rev. Biophys. 45, 345–381 (2012).
Weinberg, Z. et al. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome Biol. 11, R31 (2010).
Pleij, C.W., Rietveld, K. & Bosch, L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 13, 1717–1731 (1985).
Zhang, J. & Ferré-D'Amaré, A.R. Structure and mechanism of the T-box riboswitches. Wiley Interdiscip. Rev. RNA 6, 419–433 (2015).
Kompis, I.M., Islam, K. & Then, R.L. DNA and RNA synthesis: antifolates. Chem. Rev. 105, 593–620 (2005).
Keel, A.Y., Rambo, R.P., Batey, R.T. & Kieft, J.S. A general strategy to solve the phase problem in RNA crystallography. Structure 15, 761–772 (2007).
Ren, A., Rajashankar, K.R. & Patel, D.J. Global RNA fold and molecular recognition for a pfl riboswitch bound to ZMP, a master regulator of one-carbon metabolism. Structure doi:10.1016/j.str.2015.05.016 (25 June 2015).
Trausch, J.J., Marcano-Velazquez, J.G., Matyjasik, M.M. & Batey, R.T. Metal ion-mediated nucleobase recognition by the ZTP riboswitch. Chem. Biol. doi:10.1016/j.chembiol.2015.06.007 (2 July 2015).
Nissen, P., Ippolito, J.A., Ban, N., Moore, P.B. & Steitz, T.A. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc. Natl. Acad. Sci. USA 98, 4899–4903 (2001).
Ferré-D'Amaré, A.R. & Doudna, J.A. RNA folds: insights from recent crystal structures. Annu. Rev. Biophys. Biomol. Struct. 28, 57–73 (1999).
Putnam, C.D., Hammel, M., Hura, G.L. & Tainer, J.A. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 40, 191–285 (2007).
Rambo, R.P. & Tainer, J.A. Improving small-angle X-ray scattering data for structural analyses of the RNA world. RNA 16, 638–646 (2010).
Zhang, J., Jones, C.P. & Ferré-D'Amaré, A.R. Global analysis of riboswitches by small-angle X-ray scattering and calorimetry. Biochim. Biophys. Acta 1839, 1020–1029 (2014).
Zhang, J., Lau, M.W. & Ferré-D'Amaré, A.R. Ribozymes and riboswitches: modulation of RNA function by small molecules. Biochemistry 49, 9123–9131 (2010).
Serganov, A. & Patel, D.J. Molecular recognition and function of riboswitches. Curr. Opin. Struct. Biol. 22, 279–286 (2012).
Batey, R.T., Gilbert, S.D. & Montange, R.K. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432, 411–415 (2004).
Serganov, A. et al. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem. Biol. 11, 1729–1741 (2004).
Jones, C.P. & Ferré-D'Amaré, A.R. Crystal structure of a c-di-AMP riboswitch reveals an internally pseudo-dimeric RNA. EMBO J. 33, 2692–2703 (2014).
Ren, A. & Patel, D.J. c-di-AMP binds the ydaO riboswitch in two pseudo-symmetry–related pockets. Nat. Chem. Biol. 10, 780–786 (2014).
Gao, A. & Serganov, A. Structural insights into recognition of c-di-AMP by the ydaO riboswitch. Nat. Chem. Biol. 10, 787–792 (2014).
Ren, A. et al. Structural basis for molecular discrimination by a 3′,3′-cGAMP sensing riboswitch. Cell Reports 11, 1–12 (2015).
Kulshina, N., Baird, N.J. & Ferré-D'Amaré, A.R. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat. Struct. Mol. Biol. 16, 1212–1217 (2009).
Smith, K.D. et al. Structural basis of ligand binding by a c-di-GMP riboswitch. Nat. Struct. Mol. Biol. 16, 1218–1223 (2009).
Smith, K.D., Shanahan, C.A., Moore, E.L., Simon, A.C. & Strobel, S.A. Structural basis of differential ligand recognition by two classes of bis-(3′-5′)-cyclic dimeric guanosine monophosphate-binding riboswitches. Proc. Natl. Acad. Sci. USA 108, 7757–7762 (2011).
Montange, R.K. & Batey, R.T. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature 441, 1172–1175 (2006).
Gilbert, S.D., Rambo, R.P., Van Tyne, D. & Batey, R.T. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat. Struct. Mol. Biol. 15, 177–182 (2008).
Lu, C. et al. Crystal structures of the SAM-III/SMK riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat. Struct. Mol. Biol. 15, 1076–1083 (2008).
Johnson, J.E. Jr., Reyes, F.E., Polaski, J.T. & Batey, R.T. B12 cofactors directly stabilize an mRNA regulatory switch. Nature 492, 133–137 (2012).
Peselis, A. & Serganov, A. Structural insights into ligand binding and gene expression control by an adenosylcobalamin riboswitch. Nat. Struct. Mol. Biol. 19, 1182–1184 (2012).
Serganov, A., Huang, L. & Patel, D.J. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature 458, 233–237 (2009).
Trausch, J.J., Ceres, P., Reyes, F.E. & Batey, R.T. The structure of a tetrahydrofolate-sensing riboswitch reveals two ligand binding sites in a single aptamer. Structure 19, 1413–1423 (2011).
Huang, L., Ishibe-Murakami, S., Patel, D.J. & Serganov, A. Long-range pseudoknot interactions dictate the regulatory response in the tetrahydrofolate riboswitch. Proc. Natl. Acad. Sci. USA 108, 14801–14806 (2011).
Klein, D.J., Edwards, T.E. & Ferré-D'Amaré, A.R. Cocrystal structure of a class I preQ1 riboswitch reveals a pseudoknot recognizing an essential hypermodified nucleobase. Nat. Struct. Mol. Biol. 16, 343–344 (2009).
Liberman, J.A., Salim, M., Krucinska, J. & Wedekind, J.E. Structure of a class II preQ1 riboswitch reveals ligand recognition by a new fold. Nat. Chem. Biol. 9, 353–355 (2013).
Kang, M., Peterson, R. & Feigon, J. Structural insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Mol. Cell 39, 653–655 (2010).
Spitale, R.C., Torelli, A.T., Krucinska, J., Bandarian, V. & Wedekind, J.E. The structural basis for recognition of the PreQ0 metabolite by an unusually small riboswitch aptamer domain. J. Biol. Chem. 284, 11012–11016 (2009).
Serganov, A., Polonskaia, A., Phan, A.T., Breaker, R.R. & Patel, D.J. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature 441, 1167–1171 (2006).
Thore, S., Leibundgut, M. & Ban, N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science 312, 1208–1211 (2006).
Edwards, T.E. & Ferré-D'Amaré, A.R. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure 14, 1459–1468 (2006).
Butler, E.B., Xiong, Y., Wang, J. & Strobel, S.A. Structural basis of cooperative ligand binding by the glycine riboswitch. Chem. Biol. 18, 293–298 (2011).
Huang, L., Serganov, A. & Patel, D.J. Structural insights into ligand recognition by a sensing domain of the cooperative glycine riboswitch. Mol. Cell 40, 774–786 (2010).
Serganov, A., Huang, L. & Patel, D.J. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature 455, 1263–1267 (2008).
Garst, A.D., Heroux, A., Rambo, R.P. & Batey, R.T. Crystal structure of the lysine riboswitch regulatory mRNA element. J. Biol. Chem. 283, 22347–22351 (2008).
Baird, N.J. & Ferré-D'Amaré, A.R. Idiosyncratically tuned switching behavior of riboswitch aptamer domains revealed by comparative small-angle X-ray scattering analysis. RNA 16, 598–609 (2010).
Mellin, J.R. et al. Riboswitches: sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science 345, 940–943 (2014).
DebRoy, S. et al. Riboswitches: a riboswitch-containing sRNA controls gene expression by sequestration of a response regulator. Science 345, 937–940 (2014).
Loh, E. et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell 139, 770–779 (2009).
Xiao, H., Edwards, T.E. & Ferré-D'Amaré, A.R. Structural basis for specific, high-affinity tetracycline binding by an in vitro evolved aptamer and artificial riboswitch. Chem. Biol. 15, 1125–1137 (2008).
Kim, P.B., Nelson, J.W. & Breaker, R.R. An ancient riboswitch class in bacteria regulates purine biosynthesis and one-carbon metabolism. Mol. Cell 57, 317–328 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Schneider, T.R. & Sheldrick, G.M. Substructure solution with SHELXD. Acta Crystallogr. D Biol. Crystallogr. 58, 1772–1779 (2002).
Grosse-Kunstleve, R.W. & Adams, P.D. Substructure search procedures for macromolecular structures. Acta Crystallogr. D Biol. Crystallogr. 59, 1966–1973 (2003).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. D Biol. Crystallogr. 56, 965–972 (2000).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Keating, K.S. & Pyle, A.M. RCrane: semi-automated RNA model building. Acta Crystallogr. D Biol. Crystallogr. 68, 985–995 (2012).
Baird, N.J. & Ferré-D'Amaré, A.R. Modulation of quaternary structure and enhancement of ligand binding by the K-turn of tandem glycine riboswitches. RNA 19, 167–176 (2013).
Keller, S. et al. High-precision isothermal titration calorimetry with automated peak-shape analysis. Anal. Chem. 84, 5066–5073 (2012).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Artsimovitch, I. & Henkin, T.M. In vitro approaches to analysis of transcription termination. Methods 47, 37–43 (2009).
Acknowledgements
We thank the staff at beamlines 5.0.1 and 5.0.2 of the Advanced Light Source at Lawrence Berkeley National Laboratory for crystallographic data collection; R. Trachman for SAXS data collection; G. Piszczek (US National Heart, Lung, and Blood Institute, NHLBI, National Institutes of Health (NIH)) for isothermal titration calorimetry support; and L. Fang, S. Seifert and X. Zuo at beamline 12-ID-C of the Advanced Photon Source, Argonne National Laboratory (ANL) for SAXS support. SAXS data were collected in a core facility of the Center for Cancer Research, US National Cancer Institute (NCI) allocated under agreement between NCI and ANL (PUP-24152). We also thank S. Bachas, M. Chen, C. Fagan, M. Lau, R. Trachman, K. Warner and J. Zhang for discussions. This work was partly conducted at the ALS, on the Berkeley Center for Structural Biology beamlines, which are supported by the NIH. Use of ALS and APS was supported by the US Department of Energy. This work was supported in part by the intramural program of the NHLBI, NIH, and by a Lenfant Biomedical Fellowship to C.P.J.
Author information
Authors and Affiliations
Contributions
C.P.J. designed and carried out experiments, data analysis, diffraction data collection and structure determination. C.P.J. and A.R.F.-D. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Predicted secondary structures of the ZTP riboswitches.
(a) Halothermothrix orenii, (b) Klebsiella pneumoniae, (c) Paenibacillus sp. HGF5, (d) Spirochaeta thermophila, (e) Thermobispora bispora, (f) Thermosinus carboxydivorans, (g) Thermobacillus composti, and (h) F. ulcerans + 102-nt linker from environmental sample 3278. Secondary structures are based on the F. ulcerans secondary structure as seen in the crystal structure. Additional helices predicted to form at the 5´ end of the RNA are labeled “P0”, and the insertion domain helix of T. bispora is labeled “P5”.
Supplementary Figure 2 Crystallization of F. ulcerans ZTP riboswitch.
(a) Image of ZTP riboswitch crystals growing from a polyethylene glycol precipitate, as described in methods. Bar denotes 100 μm. (b) Denaturing polyacrylamide gel of a single riboswitch crystal. Lanes 1–3: riboswitch RNA control: 2.5, 1.25, and 0.625 μg purified RNA. Lanes 4–6: sequential crystal wash solutions. Lane 7: riboswitch crystal. (c) Density-modified 2|Fo|-|Fc| SAD map (blue mesh) used for initial model building, contoured at 2 σ.
Supplementary Figure 3 Conservation of ZTP riboswitch and mapping onto the F. ulcerans riboswitch.
(a) Conservation/covariation data published by Breaker and coworkers (Kim, P. B. et al, Mol Cell, 57, 317-28, 2015) mapped onto the sequence and secondary structure of the F. ulcerans riboswitch. Red nucleotides are more than 97% conserved, blue nucleotides are more than 90% conserved, and gray nucleotides are more than 75% conserved. (b) Cartoon view of the same conservation data mapped onto the crystallographic model. Coloring is the same as in A, except black residues (not conserved) are shown in white.
Supplementary Figure 4 Small-angle X-ray scattering (SAXS) analysis of ZTP riboswitches.
(a) Size-exclusion chromatography traces for the F. ulcerans, H. orenii, and T. carboxydivorans pfl RNAs. (b) Denaturing and native polyacrylamide gels of pooled and concentrated monomer fractions, visualized by staining with ethidium bromide. Lane 1: H. orenii. Lane 2: F. ulcerans. Lane 3: T. carboxydivorans. (c) SAXS data for the F. ulcerans, H. orenii, and T. carboxydivorans RNAs in the presence (red) and absence (black) of ZMP. Arrow indicates the presence of aggregation in the T. carboxydivorans samples without ZMP. (d) Kratky plot for the F. ulcerans riboswitch in the presence (black) and absence (red) of ZMP. (e) Cartoon view of the crystal contact interface formed between two RNA dimers (left) and overall view of the tetramer in the crystal (right). (f) Normalized size-exclusion chromatography traces of purified monomeric (left) and dimeric (right) fractions of the F. ulcerans riboswitch prior to isothermal titration calorimetry (ITC) measurements (black) and after ITC measurements (red).
Supplementary Figure 5 Representative isothermal calorimetry titration experiments and single-round transcription experiments of F. ulcerans ZTP riboswitch linker variant RNAs.
(a) 20 μM wild-type ZTP riboswitch titrated with 200 μM ZMP. (b) 50 μM +10 A linker variant titrated with 1 mM ZMP. (c) 50 μM +20 A linker variant titrated with 1 mM ZMP. (d) 50 μM +env3278 linker variant titrated with 1 mM ZMP. (e) 40 μM ZTP riboswitch lacking the pseudoknot (Δ55-75) with 40 μM 59–75 added in trans titrated with 800 μM ZMP. (f) 40 μM ZTP riboswitch Δ55-75 titrated with 800 μM ZMP. (g) Representative transcription termination experiment of F. ulcerans ZTP riboswitch linker variants. Lanes 1–3: wild-type template in the presence of 0, 100, and 1000 μM ZMP. Lanes 4–6: +10A linker template in the presence of 0, 100, and 1000 μM ZMP. Lanes 7–9: +20A linker template in the presence of 0, 100, and 1000 μM ZMP. Lanes 10–12: +env3268 linker template in the presence of 0, 100, and 1000 μM ZMP. Bands corresponding to full-length (F) and terminated (T) transcription products are indicated.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 1224 kb)
Rights and permissions
About this article
Cite this article
Jones, C., Ferré-D'Amaré, A. Recognition of the bacterial alarmone ZMP through long-distance association of two RNA subdomains. Nat Struct Mol Biol 22, 679–685 (2015). https://doi.org/10.1038/nsmb.3073
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3073
This article is cited by
-
Real-time monitoring of single ZTP riboswitches reveals a complex and kinetically controlled decision landscape
Nature Communications (2020)
-
Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure
Nature Communications (2019)
-
Alarmones as Vestiges of a Bygone RNA World
Journal of Molecular Evolution (2019)
-
Molecular prejudice: RNA discrimination against purines allows response to a cellular alarm
Nature Structural & Molecular Biology (2015)