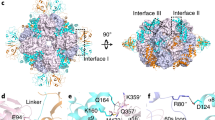
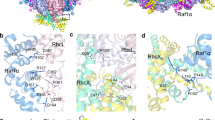
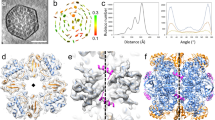
Abstract
Biogenesis of the photosynthetic enzyme Rubisco, a complex of eight large (RbcL) and eight small (RbcS) subunits, requires assembly chaperones. Here we analyzed the role of Rubisco accumulation factor1 (Raf1), a dimer of ∼40-kDa subunits. We find that Raf1 from Synechococcus elongatus acts downstream of chaperonin-assisted RbcL folding by stabilizing RbcL antiparallel dimers for assembly into RbcL8 complexes with four Raf1 dimers bound. Raf1 displacement by RbcS results in holoenzyme formation. Crystal structures show that Raf1 from Arabidopsis thaliana consists of a β-sheet dimerization domain and a flexibly linked α-helical domain. Chemical cross-linking and EM reconstruction indicate that the β-domains bind along the equator of each RbcL2 unit, and the α-helical domains embrace the top and bottom edges of RbcL2. Raf1 fulfills a role similar to that of the assembly chaperone RbcX, thus suggesting that functionally redundant factors ensure efficient Rubisco biogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Andersson, I. & Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 46, 275–291 (2008).
Hartman, F.C. & Harpel, M.R. Structure, function, regulation, and assembly of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 63, 197–234 (1994).
Ellis, R.J. Biochemistry: tackling unintelligent design. Nature 463, 164–165 (2010).
Sharkey, T.D. Estimating the rate of photorespiration. Physiol. Plant. 73, 147–152 (1988).
Portis, A.R. & Parry, M.A.J. Discoveries in Rubisco (ribulose 1,5-bisphosphate carboxylase/oxygenase): a historical perspective. Photosynth. Res. 94, 121–143 (2007).
Peterhansel, C., Niessen, M. & Kebeish, R.M. Metabolic engineering towards the enhancement of photosynthesis. Photochem. Photobiol. 84, 1317–1323 (2008).
Maurino, V.G. & Peterhansel, C. Photorespiration: current status and approaches for metabolic engineering. Curr. Opin. Plant Biol. 13, 249–256 (2010).
Whitney, S.M., Houtz, R.L. & Alonso, H. Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco. Plant Physiol. 155, 27–35 (2011).
Parry, M.A.J. et al. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 64, 717–730 (2013).
Lin, M.T., Occhialini, A., Andralojc, P.J., Parry, M.A. & Hanson, M.R. A faster Rubisco with potential to increase photosynthesis in crops. Nature 513, 547–550 (2014).
Durão, P. et al. Opposing effects of folding and assembly chaperones on evolvability of Rubisco. Nat. Chem. Biol. 11, 148–155 (2015).
Cleland, W.W., Andrews, T.J., Gutteridge, S., Hartman, F.C. & Lorimer, G.H. Mechanism of Rubisco: the carbamate as general base. Chem. Rev. 98, 549–562 (1998).
Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 59, 1555–1568 (2008).
Liu, C. et al. Coupled chaperone action in folding and assembly of hexadecameric Rubisco. Nature 463, 197–202 (2010).
Vitlin Gruber, A., Nisemblat, S., Azem, A. & Weiss, C. The complexity of chloroplast chaperonins. Trends Plant Sci. 18, 688–694 (2013).
Saschenbrecker, S. et al. Structure and function of RbcX, an assembly chaperone for hexadecameric Rubisco. Cell 129, 1189–1200 (2007).
van der Vies, S.M., Bradley, D. & Gatenby, A.A. Assembly of cyanobacterial and higher plant ribulose bisphosphate carboxylase subunits into functional homologous and heterologous enzyme molecules in Escherichia coli. EMBO J. 5, 2439–2444 (1986).
Andrews, T.J. Catalysis by cyanobacterial ribulose-bisphosphate carboxylase large subunits in the complete absence of small subunits. J. Biol. Chem. 263, 12213–12219 (1988).
Goloubinoff, P., Gatenby, A.A. & Lorimer, G.H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 337, 44–47 (1989).
Li, L.-A. & Tabita, F.R. Maximum activity of recombinant ribulose 1,5-bisphosphate carboxylase/oxygenase of Anabaena sp. strain CA requires the product of the rbcx gene. J. Bacteriol. 179, 3793–3796 (1997).
Onizuka, T. et al. The rbcX gene product promotes the production and assembly of ribulose-1,5-bisphosphate carboxylase/oxygenase of Synechococcus sp. PCC7002 in Escherichia coli. Plant Cell Physiol. 45, 1390–1395 (2004).
Bracher, A., Starling-Windhof, A., Hartl, F.U. & Hayer-Hartl, M. Crystal structure of a chaperone-bound assembly intermediate of form I Rubisco. Nat. Struct. Mol. Biol. 18, 875–880 (2011).
Emlyn-Jones, D., Woodger, F.J., Price, G.D. & Whitney, S.M. RbcX can function as a Rubisco chaperonin, but is non-essential in Synechococcus PCC7942. Plant Cell Physiol. 47, 1630–1640 (2006).
Feiz, L. et al. Ribulose-1,5-bis-phosphate carboxylase/oxygenase accumulation factor1 is required for holoenzyme assembly in maize. Plant Cell 24, 3435–3446 (2012).
Kolesinski, P., Belusiak, I., Czarnocki-Cieciura, M. & Szczepaniak, A. Rubisco accumulation factor 1 from Thermosynechococcus elongatus participates in the final stages of ribulose-1,5-bisphosphate carboxylase/oxygenase assembly in Escherichia coli cells and in vitro. FEBS J. 281, 3920–3932 (2014).
Whitney, S.M., Birch, R., Kelso, C., Beck, J.L. & Kapralov, M.V. Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by coexpressing its ancillary RAF1 chaperone. Proc. Natl. Acad. Sci. USA 112, 3564–3569 (2015).
Leitner, A. et al. The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure 20, 814–825 (2012).
Newman, J., Bränden, C.I. & Jones, T.A. Structure determination and refinement of ribulose 1,5-bisphosphate carboxylase/oxygenase from Synechococcus PCC6301. Acta Crystallogr. D Biol. Crystallogr. 49, 548–560 (1993).
Pettersen, E.F. et al. UCSF chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Chari, A. & Fischer, U. Cellular strategies for the assembly of molecular machines. Trends Biochem. Sci. 35, 676–683 (2010).
Ellis, R.J. Assembly chaperones: a perspective. Phil. Trans. R. Soc. Lond. B 368, 20110398 (2013).
Tanaka, S., Sawaya, M.R., Kerfeld, C.A. & Yeates, T.O. Structure of the RuBisCO chaperone RbcX from Synechocystis sp. PCC6803. Acta Crystallogr. D Biol. Crystallogr. 63, 1109–1112 (2007).
Kolesinski, P. et al. Insights into eukaryotic Rubisco assembly: crystal structures of RbcX chaperones from Arabidopsis thaliana. Biochim. Biophys. Acta 1830, 2899–2906 (2013).
Wheatley, N.M., Sundberg, C.D., Gidaniyan, S.D., Cascio, D. & Yeates, T.O. Structure and identification of a pterin dehydratase-like protein as a ribulose-bisphosphate carboxylase/oxygenase (RuBisCO) assembly factor in the alpha-carboxysome. J. Biol. Chem. 289, 7973–7981 (2014).
Feiz, L. et al. RAF2: a novel Rubisco biogenesis factor in maize. Plant J. 80, 862–869 (2014).
Hohmann-Marriott, M.F. & Blankenship, R.E. Evolution of photosynthesis. Annu. Rev. Plant Biol. 62, 515–548 (2011).
Kerner, M.J. et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell 122, 209–220 (2005).
Georgescauld, F. et al. GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding. Cell 157, 922–934 (2014).
Catanzariti, A.M., Soboleva, T.A., Jans, D.A., Board, P.G. & Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 13, 1331–1339 (2004).
Baker, R.T. et al. Using deubiquitylating enzymes as research tools. Methods Enzymol. 398, 540–554 (2005).
Brinker, A. et al. Dual function of protein confinement in chaperonin-assisted protein folding. Cell 107, 223–233 (2001).
Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272, 1–40 (1993).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Scheres, S.H. Semi-automated selection of cryo-EM particles in RELION-1.3. J. Struct. Biol. 189, 114–122 (2015).
van Heel, M., Harauz, G., Orlova, E.V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996).
Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 (1996).
Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003).
Götze, M. et al. Automated assignment of MS/MS cleavable crosslinks in protein 3D-structure analysis. J. Am. Soc. Mass Spectrom. 26, 83–97 (2015).
Vachette, P., Koch, M.H. & Svergun, D.I. Looking behind the beamstop: X-ray solution scattering studies of structure and conformational changes of biological macromolecules. Methods Enzymol. 374, 584–615 (2003).
Konarev, P.V., Volkov, V.V., Sokolova, A.V., Koch, M.H.J. & Svergun, D.I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 (2003).
Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 (1992).
Bernadó, P., Mylonas, E., Petoukhov, M.V., Blackledge, M. & Svergun, D.I. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 129, 5656–5664 (2007).
Petoukhov, M.V. et al. New developments in the program package for small-angle scattering data analysis. J. Appl. Crystallogr. 45, 342–350 (2012).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
Evans, P.R. Scala. Joint CCP4 ESF-EACBM Newsl. Prot. Crystallogr. 33, 22–24 (1997).
French, G. & Wilson, K. On the treatment of negative intensity observations. Acta Crystallogr. A 34, 517–525 (1978).
Sheldrick, G.M. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 479–485 (2010).
Pape, T. & Schneider, T.R. HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Crystallogr. 37, 843–844 (2004).
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 (2007).
Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. D Biol. Crystallogr. 56, 965–972 (2000).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Cowtan, K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 (2006).
Vagin, A.A. & Isupov, M.N. Spherically averaged phased translation function and its application to the search for molecules and fragments in electron-density maps. Acta Crystallogr. D Biol. Crystallogr. 57, 1451–1456 (2001).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Afonine, P.V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Kleywegt, G.T. & Jones, T.A. A super position. CCP4 ESF-EACBM Newsl. Prot. Crystallogr. 31, 9–14 (1994).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Larkin, M.A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Gouet, P., Courcelle, E., Stuart, D.I. & Metoz, F. ESPript: multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999).
Cole, C., Barber, J.D. & Barton, G.J. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36, W197–W201 (2008).
Acknowledgements
A. thaliana cDNA was a kind gift from B. Bölter (Ludwig-Maximilians-Universität München), and S. elongatus PCC7942 DNA was a kind gift from M. Hagemann (Universität Rostock). We thank A. Jungclaus for assistance with protein purification and R. Körner for performing the MS on the cross-linked samples. We also thank A. Sinz (Martin Luther University Halle-Wittenberg) for making available StavroX software for the cross-linking MS analysis. Assistance by K. Valer and J. Basquin at the Max Planck Institute of Biochemistry (MPIB) crystallization facility, as well as the staff at Swiss Light Source beamlines X10SA-PX-II and X06DA-PXIII, is gratefully acknowledged. SAXS data were collected at European Synchrotron Radiation Facility beamline BM29 with the assistance of A. Round. Cryo-EM analysis was performed in the Department of W. Baumeister at MPIB. The authors thank A. Kahraman (Universität Zürich) for helpful discussions regarding the interpretation of cross-linking data and D. Balchin and L. Popilka for critically reading the manuscript. P.W. is supported by an Emmy Noether grant of the German Research council (WE 4628/1).
Author information
Authors and Affiliations
Contributions
T.H. performed the biochemical and functional analysis of Raf1 and obtained the Raf1-domain crystals; A.B. solved the crystal structures; J.Y.B. performed the native MS and analyzed the cross-linking MS data; M.H.-H. did the SEC-MALS measurements; and G.M. and P.W. performed the cryo-EM analysis and reconstruction. M.H.-H., F.U.H. and A.B. supervised the experimental design and data interpretation, and wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Alignment of Raf1 sequences.
Amino acid sequences of a representative set of Raf1 homologs were aligned using the EBI Clustal-Ω server. Secondary structure elements for Raf1.2 from Arabidopsis thaliana are indicated above the sequences. The Raf1 domain structure is indicated by purple and orange coloring of secondary structure elements in the Raf1α and Raf1β domains, respectively. The sequences from plants (1), green algae (2) and cyanobacteria (3) are grouped separately. Similar residues are shown in red and identical residues in white on a red background. Blue frames indicate homologous regions. The consensus sequence is shown at the bottom. The chloroplast signal sequences are not shown. Asterisks below the sequence indicate mutations in Syn7942-Raf1 analyzed in this study (Fig. 4). The Uniprot accession codes for the sequences are: Q9SR19, Arabidopsis thaliana Raf1.2; Q9LKR8, Arabidopsis thaliana Raf1.1; B4FR29, Zea mays; I0YJW5, Coccomyxa subellipsoidea C-169; E1ZGR5, Chlorella variabilis; Cre06.g308450.t1.2, Chlamydomonas reinhardtii; Q00S02, Ostreococcus tauri; C1FI81, Micromonas sp. (strain RCC299 / NOUM17); B4VSU9, Coleofasciculus chthonoplastes PCC7420; Q31Q05, Synechococcus elongatus PCC7942; Q5N472, Synechococcus elongatus PCC6301; B1XK11, Synechococcus sp. PCC7002.
Supplementary Figure 2 Functional analysis of Raf1 homologs.
(a) Purified full-length Raf1 proteins and the respective α- and b-domains of Syn7942, Syn7002 and A. thaliana. AtRaf1.1/1.2 is a complex of AtRaf1.1 and AtRaf1.2 produced from a biscistronic plasmid. (b) Native-PAGE analysis of Rubisco reconstitution reactions as in Fig. 1b, containing the Raf1 proteins indicated. (c) Rubisco activities in reactions shown in (b) after supplementation with RbcS as described in Fig. 1d. Error bars, s.d. (n = 3 independent experiments). (d) Displacement of Raf1 from RbcL8 by RbcS. Purified S. elongatus RbcL8 was incubated with Syn7942-Raf1 as in Fig. 2a, followed by addition of RbcS (5 μM) and analysis by native-PAGE and immunoblotting with anti-RbcL (left) and anti-Raf1 (right). S. elongatus RbcL8 and RbcL8S8 were used as standards. (e) Stoichiometry of RbcL and Raf1 in RbcL* complexes. RbcL* was excised from native-PAGE and separated by SDS-PAGE followed by Coomassie staining and quantitation by densitometry. Top, molar ratios of RbcL and Raf1 standards as quantified by extinction coefficients. Bottom, ratios of RbcL to Raf1 stain intensities are indicated as averages ±S.D from four measurements. The molar ratio of RbcL to Raf1 in RbcL* is close 1:1. Shown is a representative Coomassie stained gel. (f) Dependence of Rubisco assembly on Raf1 concentration. Reconstitution reactions were performed as in Fig. 1e at increasing concentrations of Raf1 and the Rubisco activities obtained after 60 min are indicated as percentage of control. Error bars, s.d. (n = 3 independent experiments).
Supplementary Figure 3 SEC-MALS and SAXS analysis of Raf1 proteins.
(a) SEC-MALS analysis of purified Raf1 domains from Syn7942, Syn7002 and A. thaliana. Data showing measurements for ~30 μg of the respective proteins. Horizontal lines across the peaks indicate molar mass and homogeneity of the sample. Calculated molar masses and hydrodynamic radii are indicated. (b) Representative X-ray scattering curves of AtRaf1.2 (red), AtRaf1.2α (blue) and AtRaf1.2β (green) and Syn7942-Raf1 (black). The curves represent background-corrected averages of ten measurements. The GNOM-fitted51 curves are overlaid. (c) Density distributions for AtRaf1.2 (red), AtRaf1.2α (blue), AtRaf1.2β (green) and Syn7942-Raf1 (black) calculated with GNOM. AtRaf1.2α and AtRaf1.2β appear rod-shaped and globular, respectively. The curves for AtRaf1.2 and Syn7942-Raf1 suggests flexibly linked domains.(d) Parameters from SAXS data analysis. Radii of gyration were determined using the Guinier approximation. Scattering curves were fitted with GNOM. (e) Ensemble model for the AtRaf1.2 dimer. Two perpendicular views are shown. The backbones are represented as coils. A subset of five models matching the experimental scattering curve (Chi value 3.978) was picked from a library of 10,000 conformations by a genetic algorithm implemented in the program EOM 2.052,53. The position of the dimeric β-domain (orange) was fixed at the dyad symmetry axis. The α-domains are represented in purple; the flexible termini and inter-domain linkers are shown in gray.
Supplementary Figure 4 Functional analysis of Raf1 homologs.
(a) Native-PAGE analysis of Rubisco reconstitution reactions as in Fig. 1b, containing purified full-length Raf1 and the α- and β-domains from Syn7942 and A. thaliana. RbcS was present when indicated. (b) Rubisco activities in reactions shown in (a) after supplementation with RbcS when absent, as described in Fig. 1d. Error bars, s.d. (n = 3 independent experiments).
Supplementary Figure 5 Crystal structures of AtRaf1.2 domains.
(a,b) Experimental electron density maps for AtRaf1.2α and AtRaf1.2β. Representative regions are shown. The meshwork represents the isocontour surface at 1.0 σ level. The nominal resolutions of the AtRaf1.2α Pt-SIRAS and AtRaf1.2β Hg-SIRAS maps are 2.75 and 3.0 Å, respectively. Panel B shows a contact between two AtRaf1.2β dimers. (c) Surface properties of AtRaf1.2a. The same views as in Fig. 3c are shown. Positively and negatively charged groups are shown in blue and red, respectively. Yellow color signifies hydrophobic sidechains. (d) Superposition of three crystallographically independent copies of the AtRaf1.2β dimer. The models are represented as Cα traces. The orientation corresponds to the top-view in Fig. 3d.(e) Domain swapping in the P212121 crystal lattice of AtRaf1.2β. In this lattice the long βF-βG connecting loops reach across between adjacent dimers, making contacts to a hydrophobic pit. In the C2 crystal form, the hydrophobic residues at the loop apex fold back onto the respective hydrophobic area of the same chain, realizing an analogous intramolecular contact. Outside of the crystal lattice the conformation observed in the C2 crystal form should be strongly favored. (f) Topology of the secondary structure in the AtRaf1.2β dimer. α-Helices and β-strands are represented by cylinders and arrows, using the same color scheme as in the main text. The monomer shown in orange differs from the second by insertion of helix 12. (g) Features of the AtRaf1.2β dimer interface. On the left, the surface properties of the area facing the RbcL dimer are show using the same representation as in in panel c. On the right, one monomer is shown as backbone ribbon, the other in surface representation to reveal the AtRaf1.2β dimer interface. Yellow color signifies hydrophobic sidechains.
Supplementary Figure 6 Cross-linking coupled to mass spectrometry (CXMS).
(a) Structure of the bifunctional crosslinker disuccinimidylsuberate (DSS). The crosslinker is a 1:1 mixture of unlabeled (light; H12) and deuterium labeled (heavy; D12) compounds with a mass difference of 12.0753 Da. (b) Workflow for analysis of crosslinked protein bands marked and numbered in (c) by in-gel trypsin digestion, followed by LC–MS. (c) Crosslinking products of individual proteins S. elongatus RbcL8, Syn7942-Raf1 and Syn7002-Raf1. The purified proteins (1.25 μM RbcL8 and 10 μM of the respective Raf1 proteins) were incubated with H12:D12–DSS (2 mM) for 30 min at 25°C, followed by quenching of the crosslinking reaction with NH4HCO3 (150 mM) and analysis by SDS-PAGE. (d) Crosslinking products of RbcL8 (1.25 μM) with Syn7942-Raf1 or Syn7002-Raf1 (10 μM each). Boxed areas were analyzed as in (b). (e) Representative MS/MS spectra for the crosslinks RbcL–RbcL (Lys15–Lys460), Raf1β–RbcL (Lys344–Lys336) and Raf1β–Raf1α (Lys344–Lys188).
Supplementary Figure 7 Negative-stain EM analysis.
(a) Fourier Shell Correlation (FSC) curves of S. elongatus (Se) RbcL8–Raf14, crosslinked SeRbcL8–Raf14, and T. elongatus (Te) RbcL8–Raf14 as determined by gold standard FSC procedure in RELION-1.3. The resolution of the maps estimated by FSC with 0.5 and 0.143 correlation cut-off and no masking are given. (b) Comparison of the RbcL8–Raf14 models derived from CXMS distance restraints (Fig. 5g,h) and EM reconstruction (Fig. 6f,h,j) (assuming the C-terminal 65 residues of RbcL are structured). The backbones are represented by Cα traces. Raf1 and RbcL in the CXMS model are shown in magenta and white, respectively. Raf1 and RbcL in the EM reconstruction are shown in cyan and gray, respectively. (c) Rigid body domain fitting of SeRaf1α- and β-domains and RbcL8 missing the C-terminal 65 amino acids into the 3D reconstruction of SeRbcL8–Raf14. RbcL subunits in gray and black; Raf1α in purple and Raf1β in orange. Side- and top-views are shown. (d,e) Negative stain electron micrograph of crosslinked SeRbcL8–Raf14 (d) and of TeRbcL8–Raf14 (e). Exemplary class averages of the respective complexes obtained from 2D classification in RELION-1.3 are shown in the insets.
Supplementary Figure 8 Characterization of the RbcL–Raf1 complex of the thermophilic cyanobacterium T. elongatus.
T. elongatus RbcL and Raf1 proteins were coexpressed in E. coli and purified as a high molecular weight complex. (a) SEC-MALS analysis of RbcL-Raf1 complex in solution (~40 μg). The horizontal line across the peak indicates the calculated molar mass. Note that the sample contained a small amount of aggregated protein which leads to a higher average molar mass (~828 kDa) than expected for the RbcL8–Raf14 complex (~740 kDa). (b) nano-ESI native MS spectra of RbcL–Raf1 complex (~8 μM), Symbols indicate the charge state distributions with the charge states shown for some peaks; the calculated mass around the m/z values of the respective protein complexes is indicated. S.D. refers to the accuracy of mass values calculated from the different m/z peaks. The theoretical masses for RbcL8–Raf14 and RbcL2 are 741628.8 Da and 106265.4 Da, respectively.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 and Supplementary Note (PDF 2741 kb)
Supplementary Data Set 1
DSS cross-links identified in the complex of S. elongatus RbcL8 and Raf1 (XLSX 76 kb)
Supplementary Data Set 2
Validation of Raf1 antibodies (PDF 197 kb)
Rights and permissions
About this article
Cite this article
Hauser, T., Bhat, J., Miličić, G. et al. Structure and mechanism of the Rubisco-assembly chaperone Raf1. Nat Struct Mol Biol 22, 720–728 (2015). https://doi.org/10.1038/nsmb.3062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3062
This article is cited by
-
Rubiscosome gene expression is balanced across the hexaploid wheat genome
Photosynthesis Research (2022)
-
Scaffolding protein CcmM directs multiprotein phase separation in β-carboxysome biogenesis
Nature Structural & Molecular Biology (2021)
-
Small subunits can determine enzyme kinetics of tobacco Rubisco expressed in Escherichia coli
Nature Plants (2020)
-
Exceptionally high rates of positive selection on the rbcL gene in the genus Ilex (Aquifoliaceae)
BMC Evolutionary Biology (2019)
-
Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize
Nature Plants (2018)