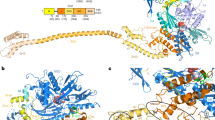
Abstract
Cotranslational chaperones, ubiquitous in all living organisms, protect nascent polypeptides from aggregation and facilitate their de novo folding. Importantly, emerging data have also suggested that ribosome-associated cotranslational chaperones have active regulatory roles in modulating protein translation. By characterizing the structure of a type of eukaryotic cotranslational chaperone, the ribosome-associated complex (RAC) from Saccharomyces cerevisiae, we show that RAC cross-links two ribosomal subunits, through a single long α-helix, to limit the predominant intersubunit rotation required for peptide elongation. We further demonstrate that any changes in the continuity, length or rigidity of this middle α-helix impair RAC function in vivo. Our results suggest a new mechanism in which RAC directly regulates protein translation by mechanically coupling cotranslational folding with the peptide-elongation cycle, and they lay the foundation for further exploration of regulatory roles of RAC in translation control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hartl, F.U. & Hayer-Hartl, M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581 (2009).
Kramer, G., Boehringer, D., Ban, N. & Bukau, B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 16, 589–597 (2009).
Preissler, S. & Deuerling, E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274–283 (2012).
Hundley, H.A., Walter, W., Bairstow, S. & Craig, E.A. Human Mpp11 J protein: ribosome-tethered molecular chaperones are ubiquitous. Science 308, 1032–1034 (2005).
Otto, H. et al. The chaperones MPP11 and Hsp70L1 form the mammalian ribosome-associated complex. Proc. Natl. Acad. Sci. USA 102, 10064–10069 (2005).
Gautschi, M., Mun, A., Ross, S. & Rospert, S. A functional chaperone triad on the yeast ribosome. Proc. Natl. Acad. Sci. USA 99, 4209–4214 (2002).
Gautschi, M. et al. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin. Proc. Natl. Acad. Sci. USA 98, 3762–3767 (2001).
Yan, W. et al. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J. 17, 4809–4817 (1998).
Nelson, R.J., Ziegelhoffer, T., Nicolet, C., Werner-Washburne, M. & Craig, E.A. The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71, 97–105 (1992).
Hundley, H. et al. The in vivo function of the ribosome-associated Hsp70, Ssz1, does not require its putative peptide-binding domain. Proc. Natl. Acad. Sci. USA 99, 4203–4208 (2002).
Huang, P., Gautschi, M., Walter, W., Rospert, S. & Craig, E.A. The Hsp70 Ssz1 modulates the function of the ribosome-associated J-protein Zuo1. Nat. Struct. Mol. Biol. 12, 497–504 (2005).
Kampinga, H.H. & Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 (2010).
Pechmann, S., Willmund, F. & Frydman, J. The ribosome as a hub for protein quality control. Mol. Cell 49, 411–421 (2013).
Willmund, F. et al. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 152, 196–209 (2013).
Chiabudini, M., Conz, C., Reckmann, F. & Rospert, S. Ribosome-associated complex and Ssb are required for translational repression induced by polylysine segments within nascent chains. Mol. Cell. Biol. 32, 4769–4779 (2012).
Rakwalska, M. & Rospert, S. The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae. Mol. Cell. Biol. 24, 9186–9197 (2004).
Muldoon-Jacobs, K.L. & Dinman, J.D. Specific effects of ribosome-tethered molecular chaperones on programmed –1 ribosomal frameshifting. Eukaryot. Cell 5, 762–770 (2006).
Liu, B., Han, Y. & Qian, S.B. Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol. Cell 49, 453–463 (2013).
Koplin, A. et al. A dual function for chaperones SSB-RAC and the NAC nascent polypeptide-associated complex on ribosomes. J. Cell Biol. 189, 57–68 (2010).
Albanèse, V., Reissmann, S. & Frydman, J. A ribosome-anchored chaperone network that facilitates eukaryotic ribosome biogenesis. J. Cell Biol. 189, 69–81 (2010).
Ducett, J.K. et al. Unfolding of the C-terminal domain of the J-protein Zuo1 releases autoinhibition and activates Pdr1-dependent transcription. J. Mol. Biol. 425, 19–31 (2013).
Prunuske, A.J., Waltner, J.K., Kuhn, P., Gu, B. & Craig, E.A. Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1. Proc. Natl. Acad. Sci. USA 109, 472–477 (2012).
Leidig, C. et al. Structural characterization of a eukaryotic chaperone—the ribosome-associated complex. Nat. Struct. Mol. Biol. 20, 23–28 (2013).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011).
Peisker, K. et al. Ribosome-associated complex binds to ribosomes in close proximity of Rpl31 at the exit of the polypeptide tunnel in yeast. Mol. Biol. Cell 19, 5279–5288 (2008).
Frank, J. & Agrawal, R.K. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322 (2000).
Frank, J., Gao, H., Sengupta, J., Gao, N. & Taylor, D.J. The process of mRNA-tRNA translocation. Proc. Natl. Acad. Sci. USA 104, 19671–19678 (2007).
Spahn, C.M. et al. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 23, 1008–1019 (2004).
Sherman, M.Y. & Qian, S.B. Less is more: improving proteostasis by translation slow down. Trends Biochem. Sci. 38, 585–591 (2013).
Shoemaker, C.J. & Green, R. Translation drives mRNA quality control. Nat. Struct. Mol. Biol. 19, 594–601 (2012).
Ingolia, N.T. Ribosome profiling: new views of translation, from single codons to genome scale. Nat. Rev. Genet. 15, 205–213 (2014).
Halic, M. et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 427, 808–814 (2004).
Shalgi, R. et al. Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 49, 439–452 (2013).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Shaikh, T.R. et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 3, 1941–1974 (2008).
Shaikh, T.R., Trujillo, R., LeBarron, J.S., Baxter, W.T. & Frank, J. Particle-verification for single-particle, reference-based reconstruction using multivariate data analysis and classification. J. Struct. Biol. 164, 41–48 (2008).
Scheres, S.H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012).
Kucukelbir, A., Sigworth, F.J. & Tagare, H.D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Buchan, D.W., Minneci, F., Nugent, T.C., Bryson, K. & Jones, D.T. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 41, W349–W357 (2013).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Jiang, J., Prasad, K., Lafer, E.M. & Sousa, R. Structural basis of interdomain communication in the Hsc70 chaperone. Mol. Cell 20, 513–524 (2005).
Lee, C.H., Kim, M.S., Chung, B.M., Leahy, D.J. & Coulombe, P.A. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat. Struct. Mol. Biol. 19, 707–715 (2012).
Trabuco, L.G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank J. Frank and H. Wang for helpful discussion and critical reading of the manuscript. We acknowledge the China National Center for Protein Sciences (Beijing) and the Explorer 100 cluster system of the Tsinghua National Laboratory for Information Science and Technology for providing computation resources. This work was supported by grants from the National Natural Science Foundation of China (31422016 and 31470722 to N.G.), the Ministry of Science and Technology of China (2010CB912402 and 2013CB910404 to N.G.; 2010CB912401 to J.L.) and the Beijing Higher Education Young Elite Teacher Project (YETP0131 to N.G.).
Author information
Authors and Affiliations
Contributions
Y.Z., J.L. and N.G. designed experiments and analyzed data. Y.Z. performed protein preparation, ribosome purification (together with C.M. and S.W.), data collection (together with Y.Y.), image processing (together with N.L.) and spot assays. J.Z., C.C. and L.Y. contributed to yeast-strain and plasmid construction. Y.Z. and N.G. wrote the manuscript; L.Y. commented on the manuscript; and all authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Structural overview of the S. cerevisiae 80S ribosome bound with Zuotin and Ssz.
(a-c) Cryo-EM density maps of the empty 80S (a), 80S-Zuotin (b) and 80S-RAC (c) complexes are shown in surface representation, with view direction centered at the PTE (peptide tunnel exit) (a-d). The 60S, 40S, Zuotin, and RAC are colored cyan, yellow, red and orange, respectively. NTD and CTD denote the N- and C-terminal domains of Zuotin. (d) For the 80S-RAC map, densities of Zuotin (red), Ssz (purple) and ES27 (green) were segmented and separately colored. The two maps (b and c) were selected from a large number of similar structures (datasets Zuo1 and RAC6, see Supplementary Table 1), based on their relatively high occupancy of RAC or Zuotin on the ribosome. (e-h) Similar as (a-d), but displayed in a classical front view. (i and j) Overview (i) and close-up view (j) of the interaction of Zuotin with Ssz. The putative densities of Ssz are colored transparent purple, with an atomic model of full-length Ssz (see online methods) docked in its densities (cross-correlation coefficient 0.8329). The map is from the dataset RAC6 (Supplementary Table 1) and filtered to 12 Å. The fitting suggests that Ssz-NTD directly interacts with Zuotin-NTD, which is consistent with previous data that Ssz-CTD does not stably associate with Zuotin3 and is dispensable for its in vivo function4.
Supplementary Figure 2 Conformational dynamics of ES27 on the 80S ribosome.
(a,d) Two distinct conformations of ES27 in the cryo-EM structures of the empty 80S ribosome. The two maps (filtered to 12 Å) are from the dataset of RAC1 (Supplementary Table 1). ES27, as an inherent dynamic component of the 25S rRNA, has two predominant states, with the helix stem toward the PTE (peptide tunnel exit) (a) or L1 stalk (d). (b,e) Two representative cryo-EM structures of the 80S-RAC complex, with ES27 in different conformations. The two maps (filtered to 10 Å) are from datasets RAC2 (b and c) and RAC11 (e and f). (c,f) Same as (b) and (e), with view directions centered at RAC. The 60S, 40S, RAC and ES27 are colored cyan, yellow, orange and green, respectively. ES27 is a dynamic rRNA helix, which can exist in sharply different conformations5, and has been proposed to have a possible role in regulating the binding of various co-translational factors at the PTE5-8. Our structural analysis shows that the RAC binding is independent of the conformational state of ES27, which suggests that ES27 might regulate the binding of Ssb (Supplementary Fig. 1), given the possible spatial relationship of ES27 and Ssb at the PTE.
Supplementary Figure 3 The workflow of the 3D image classification used in image processing.
The schematic diagram of a cascade of 3D classification procedures based on RELION is shown (see online methods for full details). (1) 318,404 particles in the nonrotated state (from three datasets with relatively higher factor occupancy) were first combined and subjected to refinement, which resulted in a 4.9 Å map. (2) The particles were subjected to further 3D classification using RELION to remove those with slightly rotated 40S subunit, and only particles in -1.5 to -3.5 μm defocus range were kept. The resulting 167,534 particles rendered a 5.2 Å map after structural refinement. (3) To further improve the occupancy of factors, particles were subjected to a multiple reference classification (see online method) and split into two classes. (4) One class with higher occupancy of factors, with a number of 75,055 particles, was kept for another round of multiple reference classification. (5) The final 24,619 particles after two rounds of multiple reference classification (Step 3 and 4) were subjected to refinement, and a 7.2 Å map was obtained. The estimated resolutions were calculated according to the gold-standard FSC 0.143 criterion (Supplementary Fig. 6). Bfactor denotes the procedure of B-factor sharpening. The area outlined by pink lines is expected location of factor binding.
Supplementary Figure 4 Sequence analysis of Zuotin MD and ES12.
(a) Multiple sequence alignment of Zuotin-MD. Residues are colored based on their sequence conservation. The secondary structure of Zuotin-MD is also shown as a single long helix (red), with selected residue positions labeled with numbers and sequences involved in the 60S and 40S binding indicated by brackets. The numbering is based on S. cerevisiae. The sequence alignment was created using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). (b) Cartoon showing the length of ES12 in the corresponding species in (a). An evident observation is that the length of Zuotin-MD correlates with the length of ES12, indicating a co-evolution pattern.
Supplementary Figure 5 Comparison of RAC and signal peptide–recognition particle (SRP) on the 80S ribosome.
(a,b) Overview (a) and closed-up view (b) of the cryo-EM map of the 80S-SRP complex (EMDB 1217)9. SB, stalk base; BK, beak; CP, central protuberance; H43 and H44, corresponding helices of the 25S rRNA; h5, h14 and h15, helices of the 18S rRNA; Alu, Alu domain of SRP. The 40S:SRP contact is indicated by a red circle. The map of the 80S-SRP complex is filtered to 10 Å. (c) Cryo-EM map of the 80S-RAC complex, showing in the same view as (a). RAC is colored orange and the 40S:RAC contact is indicated by a red circle. The map of the 80S-RAC complex is filtered to 10 Å (the dataset of RAC6). (d) Superimposition of (a) and (c), showing in a view centered at the PTE. RAC and SRP are apparently incompatible on the 80S ribosome.
Supplementary Figure 6 Local resolution maps and FSC curves of the three nonrotated 80S–RAC density maps.
(a-e, g-k, and m-q) Local resolution maps of the 4.9 Å (a-e), 5.2 Å (g-k) and 7.2 Å (m-q) density maps of the nonrotated 80S-RAC complex are colored according to the color scale on the right. The maps are displayed in classical front view (a-b, g-h and m-n) and a viewed centered on RAC (c-e, i-k, and o-q). Respective central slices of the local resolution maps are also shown (b, d, h, j, n, and p). Due to the substoichiometric binding and the flexibility of factors, local resolution maps are displayed at low contour levels (e, k and q), in order to show factor densities. As shown, with the increase of sample homogeneity (from e, k to q), local resolution at the factor region improves as well, although the overall resolution of the density maps becomes worse. (f, l and r) Gold-standard fourier shell correlation (FSC) curves of the three density maps.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 16749 kb)
Cryo-EM structure of the yeast 80S–RAC complex in 10.2-Å resolution.
The density map is from the dataset RAC6, which has relatively highest occupancies for both Zuotin and Ssz. The map is displayed in surface representation with segmented parts separately colored. The movie shows the overall orientation of Zuotin and Ssz on the ribosome. (MOV 5168 kb)
The 4.9-Å cryo-EM map of the yeast 80S–RAC complex in nonrotated state.
The density map is obtained by structural refinement of multiple datasets based on three-dimensional classification. The map shows the quality of the reconstruction. Due to substoichiometric binding and flexibility of factors, which results in fragmentation of factors in high-resolution structures, segmented map of RAC is presented in a lower contour level. After two rounds of RELION-based multiple-reference refinement, a 7.2 Å cryo-EM map of the yeast 80S-RAC complex in nonrotated state was obtained. Three ribosomal contacts (C1, C2 and C3) of RAC are shown, with five ribosomal components highlighted. (MOV 4413 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Ma, C., Yuan, Y. et al. Structural basis for interaction of a cotranslational chaperone with the eukaryotic ribosome. Nat Struct Mol Biol 21, 1042–1046 (2014). https://doi.org/10.1038/nsmb.2908
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2908
This article is cited by
-
Pathway of Hsp70 interactions at the ribosome
Nature Communications (2021)
-
The ribosome-associated complex RAC serves in a relay that directs nascent chains to Ssb
Nature Communications (2020)
-
Structural insights into a unique Hsp70-Hsp40 interaction in the eukaryotic ribosome-associated complex
Nature Structural & Molecular Biology (2017)
-
Cryo-EM structures of the 80S ribosomes from human parasites Trichomonas vaginalis and Toxoplasma gondii
Cell Research (2017)
-
Structural basis of HypK regulating N-terminal acetylation by the NatA complex
Nature Communications (2017)