Abstract
Polo-like kinase 4 (Plk4) is a key regulator of centriole duplication, an event critical for the maintenance of genomic integrity. We show that Plk4 relocalizes from the inner Cep192 ring to the outer Cep152 ring as newly recruited Cep152 assembles around the Cep192-encircled daughter centriole. Crystal-structure analyses revealed that Cep192- and Cep152-derived peptides bind the cryptic polo box (CPB) of Plk4 in opposite orientations and in a mutually exclusive manner. The Cep152 peptide bound to the CPB markedly better than did the Cep192 peptide and effectively 'snatched' the CPB away from a preformed CPB–Cep192 peptide complex. A cancer-associated Cep152 mutation impairing the Plk4 interaction induced defects in procentriole assembly and chromosome segregation. Thus, Plk4 is intricately regulated in time and space through ordered interactions with two distinct scaffolds, Cep192 and Cep152, and a failure in this process may lead to human cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gönczy, P. Towards a molecular architecture of centriole assembly. Nat. Rev. Mol. Cell Biol. 13, 425–435 (2012).
Brito, D.A., Gouveia, S.M. & Bettencourt-Dias, M. Deconstructing the centriole: structure and number control. Curr. Opin. Cell Biol. 24, 4–13 (2012).
Nigg, E.A. & Stearns, T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 13, 1154–1160 (2011).
Ganem, N.J., Godinho, S.A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Holland, A.J. & Cleveland, D.W. Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 13, 501–514 (2012).
Nigg, E.A. & Raff, J.W. Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 (2009).
O'Connell, K.F. et al. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105, 547–558 (2001).
Bettencourt-Dias, M. et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 15, 2199–2207 (2005).
Habedanck, R., Stierhof, Y.D., Wilkinson, C.J. & Nigg, E.A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7, 1140–1146 (2005).
Delattre, M., Canard, C. & Gönczy, P. Sequential protein recruitment in C. elegans centriole formation. Curr. Biol. 16, 1844–1849 (2006).
Pelletier, L., O'Toole, E., Schwager, A., Hyman, A.A. & Müller-Reichert, T. Centriole assembly in Caenorhabditis elegans. Nature 444, 619–623 (2006).
Dzhindzhev, N.S. et al. Asterless is a scaffold for the onset of centriole assembly. Nature 467, 714–718 (2010).
Kim, T.-S. et al. Hierarchical recruitment of Plk4 and regulation of centriole biogenesis by two centrosomal scaffolds, Cep192 and Cep152. Proc. Natl. Acad. Sci. USA 110, E4849–E4857 (2013).
Sonnen, K.F., Gabryjonczyk, A.M., Anselm, E., Stierhof, Y.D. & Nigg, E.A. Human Cep192 and Cep152 cooperate in Plk4 recruitment and centriole duplication. J. Cell Sci. 126, 3223–3233 (2013).
Lawo, S., Hasegan, M., Gupta, G.D. & Pelletier, L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat. Cell Biol. 14, 1148–1158 (2012).
Sonnen, K.F., Schermelleh, L., Leonhardt, H. & Nigg, E.A. 3D-structured illumination microscopy provides novel insight into architecture of human centrosomes. Biol. Open 1, 965–976 (2012).
Kitagawa, D. et al. Structural basis of the 9-fold symmetry of centrioles. Cell 144, 364–375 (2011).
van Breugel, M. et al. Structures of SAS-6 suggest its organization in centrioles. Science 331, 1196–1199 (2011).
Leung, G.C. et al. The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat. Struct. Biol. 9, 719–724 (2002).
Slevin, L.K. et al. The structure of the plk4 cryptic polo box reveals two tandem polo boxes required for centriole duplication. Structure 20, 1905–1917 (2012).
Elia, A.E. et al. The molecular basis for phospho-dependent substrate targeting and regulation of Plks by the polo-box domain. Cell 115, 83–95 (2003).
Cizmecioglu, O. et al. Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. J. Cell Biol. 191, 731–739 (2010).
Hatch, E.M., Kulukian, A., Holland, A.J., Cleveland, D.W. & Stearns, T. Cep152 interacts with Plk4 and is required for centriole duplication. J. Cell Biol. 191, 721–729 (2010).
Yun, S.M. et al. Structural and functional analyses of minimal phosphopeptides targeting the polo-box domain of polo-like kinase 1. Nat. Struct. Mol. Biol. 16, 876–882 (2009).
Ko, M.A. et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat. Genet. 37, 883–888 (2005).
Zhao, H. et al. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 15, 1434–1444 (2013).
Dix, C.I. & Raff, J.W. Drosophila Spd-2 recruits PCM to the sperm centriole, but is dispensable for centriole duplication. Curr. Biol. 17, 1759–1764 (2007).
Lin, Y.C. et al. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J. 32, 1141–1154 (2013).
Inanç, B. et al. Abnormal centrosomal structure and duplication in Cep135-deficient vertebrate cells. Mol. Biol. Cell 24, 2645–2654 (2013).
Kleylein-Sohn, J. et al. Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 (2007).
Johmura, Y. et al. Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc. Natl. Acad. Sci. USA 108, 11446–11451 (2011).
Lee, K.S., Yuan, Y.-L., Kuriyama, R. & Erikson, R.L. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol. Cell. Biol. 15, 7143–7151 (1995).
Sillibourne, J.E. et al. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol. Biol. Cell 21, 547–561 (2010).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Kang, Y.H. et al. Mammalian polo-like kinase 1-dependent regulation of the PBIP1–CENP-Q complex at kinetochores. J. Biol. Chem. 286, 19744–19757 (2011).
Liu, F. et al. Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat. Chem. Biol. 7, 595–601 (2011).
Acknowledgements
We are grateful to V. Barr and S. Garfield for critical reading of the manuscript and B. Chhun and E. Shumsky for technical assistance on 3D-SIM. This work was supported in part by intramural research grants of the US National Cancer Institute (K.S.L.) and National Institute of Diabetes and Digestive and Kidney Diseases (W.Y.); National Research Foundation of Korea (NRF) Global Research Laboratory program grant K20815000001 (B.H.O.); NRF grant 2011-0030027 (S.J.K.); NRF National Leap Research Program (no. 2010-0029233) and Global Frontier Project (NRF-M1AXA002-2012M3A6A4054949) grants (K.W.L.); Korea Basic Science Institute grant T33418 (J.K.B.); World Class Institute program grant 2009-002 (B.Y.K.) funded by the Ministry of Science, ICT and Future Planning of the Republic of Korea; Next-Generation BioGreen 21 Program grant PJ009594 (N.-H.K.) from the Rural Development Administration, Republic of Korea; and a grant-in-aid for scientific research (23380065) from the Ministry of Education, Sports, Science and Technology of Japan (H.H.).
Author information
Authors and Affiliations
Contributions
Experiments were designed and data interpreted by S.-Y.P., J.-E.P., T.-S.K., J.H.K., M.-J.K., B.K., L.T., R.N.M., M.A., S.K., K.W.L. and R.L.E. Crystal structures of the apo-CPB structure (J.-H.K. and B.K.), the CPB–Cep192-58mer complex (S.-Y.P., L.T., J.H.K., B.K. and J.-E.P.) and the CPB–Cep152-60mer complex (S.-Y.P., L.T., M.-J.K., B.K. and J.-E.P.) were determined independently. FP, 3D-SIM and cell-based analyses were performed by J.-E.P., and all biochemical analyses were performed by T.-S.K. In addition, N.-H.K., B.Y.K., J.K.B., H.H. and K.W.L. provided reagents and resources, and K.S.L., Y.W., B.-H.O. and S.J.K. designed the experiments, interpreted the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
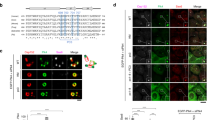
Supplementary Figure 1 Analyses of subcentrosomal localizations for Cep192, Cep152 and Plk4.
(a) U2OS cells immunostained with Alexa 488 (green)-conjugated Cep192 N-terminal (N) and Alexa 647 (magenta)-conjugated Cep192 C-terminal (C) antibodies were subjected to 3D-SIM microscopic analysis, as described in the Methods. Two representative images used for analyses were provided. The lines (a’ and b’) were drawn arbitrarily and their intensity line profiles (bottom left) were generated using the Zeiss ZEN software. The outer diameters of Cep192 (N) and (C) rings were quantified from G1 and S cells (n=44) (graph). Unevenly shaped toroids were excluded from quantification. Scale bars, 0.5 μm; error bars, s.d. (b) An example of measuring the diameter of Plk4 ring structure from either a daughter centriole without recruited Cep152 (a’) or a mother centriole with already recruited Cep152 (b’). The image is from the Figure 1a, 1st panel. Cep192 ring structures were found to be associated with both mother and daughter centrioles at all times. The data quantified from the outer diameters of Plk4 rings are shown in Figure 1b. Scale bar, 0.5 μm.
Supplementary Figure 2 Human Plk4 CPB (580–808) fragment forms a stable homomeric complex and is sufficient to interact with Cep192 and Cep152 in a phosphoindependent manner.
(a) A set of 293T cells was individually transfected with either HA-Cep192 (left) or HA-Cep152 (right), HA-Plk4, FLAG-Plk4, or control vector (–), where indicated, and the resulting lysates were mixed (Mix) prior to anti-Flag co-immunoprecipitation (IP) analysis. A second set of cells was co-transfected (co-Tfx) with the indicated constructs (“–“ denotes empty vector) and the resulting lysates were subjected to anti-FLAG co-immunoprecipitation analysis under the same conditions. Note that immunoprecipitation of FLAG-Plk4 co-precipitates HA-Plk4 under the co-transfected, but not mixed, conditions, suggesting that once a Plk4 homomeric complex is generated as Plk4 is expressed, it hardly exchanges its subunit with another molecule of Plk4. Asterisks, cross-reacting proteins with Cep192 or Cep152 immunoprecipitates; arrows, co-immunoprecipitated HA-Plk4 that migrates a little faster than a cross-reacting protein (asterisk). (b) 293T cells cotransfected with the indicated constructs were treated with thymidine (Thy) or nocodazole (Noc). The resulting lysates were incubated with either buffer alone (–) or λ-phosphatase (λ-PPase), and then subjected to co-immunoprecipitation (IP) analysis. Endogenous Nedd1, which is phosphorylated during mitosis1, was immunoblotted to assess the efficiency of λ-phosphatase treatment under our experimental conditions. Note that λ-phosphatase treatment failed to alter the level of Plk4 interacting with Cep192 or Cep152. p-Nedd1, phosphorylated Nedd1. (c) MBP pull-downs were performed using bacterially expressed, purified proteins. Arrows indicate the full-length forms of MBP-fused Cep192 or Cep152. (d) 293T cells cotransfected with the indicated FLAG- or HA-tagged Plk4 constructs were subjected to immunoprecipitation (IP) analysis. Note that, when compared with other longer forms, the FLAG-Plk4 CPB (580–808) construct possessed the full capacity to interact with the Plk4 C-terminal region (C) (580–970) or other CPB variants. The PB3 (854–970), which is reported to form a homodimeric complex 2, showed a detectable level of interaction with another PB3 molecule only after a long exposure (expo.). (e) 293T cells cotransfected with various FLAG-Plk4 constructs and GFP-fused Cep192 (201–280) or Cep152 (1–217) 3 were subjected to immunoprecipitation (IP) analysis. (f) To determine the core region of Cep152 critically required for Plk4 CPB binding, 293T cells cotransfected with FLAG-Plk4 CPB (580–808) and various GFP-Cep152 (1–217) truncated forms were subjected to immunoprecipitation (IP) analysis. (g) 293T cells cotransfected with the indicated constructs were subjected to immunoprecipitation analysis. To achieve stable overexpression, a kinase-inactive Plk4 K41M mutant was used. The immunoprecipitates were analyzed by SDS-PAGE and stained with Coomassie (CBB). Arrows, coimmunoprecipitated FLAG-Plk4; Asterisk, cross-reacting protein; H.C., IgG heavy chain; L.C., IgG light chain. (h) 293T cells cotransfected with the indicated constructs were subjected to immunoprecipitation analysis. ∆58, Cep192 lacking residues 201–258. (i) 293T cells transfected with the indicated constructs were analyzed similarly as in (g). Arrows indicate FLAG-Plk4 coimmunoprecipitated with the GFP ligands. Asterisk, cross-reacting protein; H.C., IgG heavy chain; L.C., IgG light chain.
Supplementary Figure 3 The binding mode of the CPB–Cep192-58mer and the CPB–Cep152-60mer complexes.
(a) Analysis of the crystal structure of human apo-Plk4 CPB (PDB: 4N9J) revealed a dimeric interface formed between subunit A PB2 and subunit B PB2. The dimeric interface comprises four main chain hydrogen bonds (two reciprocal hydrogen bonds between the P800 of one subunit and the G804 of the other subunit, and a pair of hydrogen bonds between the two I802 residues from each subunit) (Top view), 2 additional hydrogen bonds between the Y705 of β7 and the A796 carbonyl of the α2-β13 loop (Top view), and multiple hydrophobic interactions (Y705, I778, L782, I785, I786, F798, I801, I802 and I803) between two β13 strands and also between two α2 helices (Side view). Within a subunit, the PB1 and PB2 domains are linked by evolutionarily conserved S700 and P701 residues. Remarkably, a pair of PB1-PB2 intersubunit hydrogen bonds is evident between the R598 carbonyl from the PB1 of subunit A and the R805 guanidyl from the PB2 of subunit B (Top view). These unique intersubunit interactions involving both PB1 and PB2 domains from two different subunits (i.e., subunit A and subunit B) may function like “a diagonal supporting beam of a triangular bracket” that stabilizes the “X-shaped” CPB structure by reinforcing not only the intersubunit PB2-PB2 junctional interaction but also the intrasubunit PB1-PB2 bridging (S700-P701) interaction. (b) Superposition of the structure of Homo sapience (H. s.) CPB (PDB: 4N9J) with that of Drosophila melanogaster (D. m.) CPB (PDB: 4G7N) revealed that an inserted sequence present in human Plk4 CPB clashed with the PB2 α2-helix of Drosophila CPB (red dotted box). The residues lacking in Drosophila CPB are indicated by dashed lines (bottom). The root-mean-square-deviation (r.m.s.d.) of superposed PB1-PB2 Cα atoms between human CPB (residues 586–807) and Drosophila CPB (residues 379–596) is 1.94 Å across 196 aligned residues (39.3% identity). Arrow and cylinder above the aligned sequences indicate β-strand and α-helix, respectively. Numbers indicate the positions of residues in the human Plk4 primary sequence. Identical residues are marked by ‘*’. Strongly conserved and weakly conserved residues are indicated by ‘:’ and ‘.’, respectively. The superposition was performed by using the WinCoot’s secondary structure matching program 4, and the sequence alignment was carried out using the ClustalW2 program 5. (c) The crystal structures of the CPB apo-form (gray) and the CPBs bound to Cep192-58mer (magenta) or Cep152-60mer (green) were superposed and shown as Cα trace. The CPB residues whose positions were greatly altered upon binding to Cep192-58mer or Cep152-60mer are indicated. The r.m.s.d. values for superposed PB1-PB2 Cα atoms of a subunit of the CPB dimer are 0.87 Å between apo-CPB and CPB-Cep192-58mer and 1.0 Å between apo-CPB and CPB-Cep152-60mer. Inset shows overall binding modes of the 58mer and the 60mer to CPB. (d) The crystal structure of the Plk4 CPB–Cep192-58mer complex was superposed with that of the Plk1 PBD–PLHSpT complex (PDB: 3HIK) 6. Note that Plk1 PBD interacts with a phospho-peptide, PLHSpT, predominantly through the β1 of PB1 and the β8 and β9 of PB2 6. On the other hand, Plk4 CPB interacts with the 58mer peptide primarily through the α1 and β1 of PB1 and the β7 of PB2. The r.m.s.d. between Plk1 PB1 (residues 412–502) and Plk4 PB1 (residues 586–700) is 1.85 Å across 75 aligned residues (14.7% identity). (e) The interactions between CPB (orange) and the α-helical region of Cep192-58mer (magenta) and Cep152-60mer (green) are shown. Oxygen and nitrogen atoms are colored in red and blue, respectively. Hydrogen bonds important for the interaction are depicted as black dashed lines. In the case of the 58mer binding (left), the residues from its α-helix region were engaged in the hydrophobic interactions with the α1 and β1 of PB1 (i.e., the Y230 and L234 residues of the 58mer with the I602, K600 aliphatic portion, and P701 of the PB1, and the F222, Y223, L227, and F231 residues of the 58mer with the Y688, F692, L695, V696, and K699 aliphatic portion of the PB1). Additionally, the hydrogen bonds formed between the hydroxyl group of Y230 and the carbonyl of K600, and among D224, H226 (NH in the backbone), and Q604 residues stabilized the helical interactions. In the case of the 60mer binding (right), the residues from its α-helix were engaged in the multiple layers of well-arranged hydrophobic interactions with the residues from the CPB α1-helix, thus forming a highly stable antiparallel coiled-coil dimer between the two α-helices. The R26 aliphatic portion, L30, L33, L34, L37 and P38 from the 60mer formed prominent hydrophobic interactions with the K699 aliphatic portion, I602, V696, L695, F692, and Y688 (in order) from the α1-helix. Hydrogen bonds (Q31 with Q604 and H39 with T606) appeared to reinforce the multiple hydrophobic interactions formed between the two α-helices. (f) The interactions between CPB and the D-rich motif of Cep192-58mer (left) or Cep152-60mer (right) are depicted. Oxygen and nitrogen atoms are indicated in red and blue, respectively. The overall electron density was weak in this region to define specific interactions. The D-rich motifs of both Cep192-58mer and Cep152-60mer are surrounded by one arginine and five lysine residues (K608, K625, K634, K681, R684, and K685; rectangled in the figure), forming a crater-like structure. The three consecutive D214, D215, and D216 residues in the D-rich motif of the 58mer could engage in electrostatic interactions (dashed lines) with the K/R residues in the crater (D214 with K685 and D216 with R684) (left). In addition, the hydrophobic residue, I217, submerged (somewhat shallowly) into a hydrophobic pocket generated by the A609, V621, L623, K685 aliphatic portion, and Y688 from the PB1 of CPB. In the case of the 60mer, the D44 residue in the D-rich motif could be involved in an electrostatic interaction (dashed line) with K685 (right). This interaction was further stabilized by the L42 that was well embedded into the hydrophobic pocket at the center of the PB1 crater (right). (g,h) Surface representations of the overall structures of human apo-CPB (top) or CPB in complex with Cep192-58mer (left) or Cep152-60mer (right) are shown in both side (g) and top view (h). Surface colors represent electrostatic potentials depicted at the same scale (from −66.4 to +66.4) in all cases (red, negative; blue, positive; white, neutral). (g) In the apo-CPB, the K/R crater formed by surrounding one arginine and five lysine residues is indicated. The negatively charged residues from the 58mer D-rich motif appear to be effectively negated by the positive charged residues from the K/R crater. The binding of the 60mer modestly alters the electrostatic potential of the K/R crater, resulting in an enhanced exposure of nonpolar surface. Regardless of these changes, the D-rich motif-dependent interactions for both the 58mer and the 60mer are critically required for proper CPB binding (see Figure 3c,d). (h) In the apo-CPB, the presence of a basic patch (dotted circle) composed of six lysine and two arginine residues (symmetrically located pairs of K600, K711, R805, and K806 residues; the residues contributed from each subunit are colored in white or orange) is evident over the dimeric interface. Unlike Cep192-58mer, Cep152-60mer possesses a unique N-terminal acidic sequence motif (residues from E15 to E21) that interacts with the basic patch residues by effectively neutralizing the electrostatic potential of this region. Binding of the 60mer altered the position of R805 and K806 present at the C-terminus of CPB (Supplementary Figure 3c). The positive dipole of the α-helix in the 60mer was also neutralized by the negatively charged side chains of its own. In contrast, the 58mer lacking an analogous acidic sequence failed to induce these effects. However, the neutralizing effect was in part achieved by the negative helical dipoles of two 58mer peptides aligned along the dimeric interface.
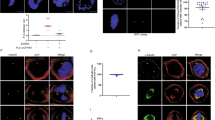
Supplementary Figure 4 Characterization of cancer-associated Cep152 E21K and V8A mutations impaired in Plk4 binding.
(a–c) The CEP152 E21K mutation found in human colon and rectum adenocarcinoma [Catalogue of Somatic Mutations in Cancer (http://www.sanger.ac.uk/cosmic)] induces a defect in procentriole assembly. U2OS stably expressing either control vector or siRNA-resistant CEP152-sil or CEP152-sil E21K mutant (no tag) were silenced for either control Luciferase (GL siRNA) or CEP152 (CEP152 siRNA). (a) The resulting cells were subjected to immunoblotting analysis using the levels of actin as loading controls. (b,c) The cells in (a) were immunostained with anti-Cep152 and anti-Sas6 antibodies, and the fraction of interphase cells with centrosomally localized Sas6 (b) and the fraction of mitotic cells with abnormal (misaligned and lagging; arrows) chromosome morphologies (c) were quantified from three independent experiments. The results were provided in Figure 3f,g. Representative images used for quantification were shown. Scale bars in (b,c), 10 μm. (d–h) The CEP152 V8A mutation found in human ovarian serous cystadenocarcinoma [Catalogue of Somatic Mutations in Cancer (http://www.sanger.ac.uk/ cosmic)] induces a defect in procentriole assembly and chromosome segregation. (d) 293T cells cotransfected with the indicated constructs were subjected to immunoprecipitation (IP) analyses. Numbers, relative signal intensities. (e) U2OS stably expressing either control vector or siRNA-resistant CEP152-sil or CEP152-sil V8A mutant (no tag) were silenced for either control Luciferase (GL siRNA) or CEP152 (CEP152 siRNA), and then analyzed as in Supplementary Figure 4a–c. (f) The cells in (e) were immunostained with anti-Cep152 and anti-Sas6 antibodies. Representative images are shown. Scale bars, 10 μm. (g,h) From the samples obtained in (f), the number of centrosomal Sas6 signals (from 0 to ≥3) among interphase cells (g) and the number of mitotic cells with aberrant chromosomal morphologies (i.e., misaligned and lagging as shown in Supplementary Figure 4a–c) (h) were quantified from three independent experiments (≥200 cells/cell line/experiment). Bars, s.d.
Supplementary Figure 5 The incompatible nature of Cep192-58mer and Cep152-60mer binding to Plk4 CPB.
(a) MBP-CPB, MBP-Cep152-60mer, and MBPL-Cep192-58mer (MBPL, a 30 residue-longer MBP variant, was needed to differentiate the size of MBPL-Cep192-58mer from that of MBP-Cep152-60mer) were mixed and subjected to gel filtration chromatography. Fractions were collected and analyzed by 10% SDS-PAGE. The gel was stained with Coomassie (CBB) and the proteins in each fraction were quantified (graphs). Arrows indicate the peak fraction (fraction #14) containing the highest amounts of the MBP-CPB–MBP-Cep152-60mer complex. Note that, unlike MBP-Cep152-60mer, MBPL-Cep192-58mer hardly forms a complex with MBP-CPB. (b) To corroborate the incompatible nature of Cep192 and Cep152, we also used the two previously identified Plk4-binding fragments, Cep192 (201–280) and Cep152 (1–217) 3 (see Supplementary Figure 2e), expressed as MBP-fused forms, to carry out GST pull-downs with GST-Plk4 C-terminal (580–970) fragment (C). After incubating the purified proteins as indicated, bead-associated proteins were washed, separated by 10% SDS-PAGE, and stained with Coomassie (CBB). Arrowheads indicate full-length proteins with their expected sizes. Several cleavage products for Cep192 (201–280) and Cep152 (1–217) were evident. “–“ indicates control buffer. Note that either Cep192 (201–280) or Cep152 (1–217) alone bound to Plk4 C efficiently (lanes 6 and 7). However, in the presence of both Cep192 (201–280) and Cep152 (1–217) in the same binding mix, the level of Cep152 (1–217) binding to the Plk4 C was unaffected, whereas that of Cep192 (201–280) binding to the Plk4 C was completely disrupted (lane 8). (c,d) 293T cells were singly transfected with either FLAG-Plk4, GFP-Cep192, or GFP-Cep152. Lysates were mixed in such a way that either GFP-Cep152 (c) or GFP-Cep192 (d) was gradually increased as indicated (black right-angled triangle). After immunoprecipitating FLAG-Plk4, coimmunoprecipitated Cep192 and Cep152 were analyzed. “–“ indicates lysates transfected with control vector. Note that in the presence of equal amounts of Cep192 and Cep152 in the input, Cep152 outcompeted Cep192 in binding to Plk4 (the 4th lanes in c and d). When Cep192 was expressed several-fold higher than Cep152, Cep192 competitively bound to Plk4 at a level similar to that of Cep152 (the 6th lane in d). (e) The overall structure of the CPB–Cep192-58mer complex is shown with a close-up view of the tetrameric interface between the 58mer C-termini and the PB2-PB2 junction. Oxygen and nitrogen atoms are colored red and blue, respectively. The C-terminal regions of two 58mers bound to each subunit of the CPB dimer were arranged along the dimeric junction in reverse orientation, forming symmetrical hydrophobic interactions among P237, I240, and Y241 residues. In addition, the P237, I240, and Y241 residues also formed reciprocal hydrophobic interactions with I802, F706, and the R805 aliphatic portion from the β7 and β13 of PB2. These 58mer-58mer and 58mer-PB2 interactions may help promote the formation of the 2:2 CPB–58mer complex. (f) The 60mer binds to a CPB dimer in such a way that its N-terminal acidic residues (D20 and D23) interact with the K711 residue (orange) of a CPB subunit that it binds to (Cα trace in orange) and further extends toward the K711 residue (highlighted in blue) in the other subunit (Cα trace in blue). Since the K711 residue plays a critical role in binding to Cep152-60mer (see Figure 3d), the steric occlusion of the second K711 residue (blue) may prevent another 60mer from binding to the other CPB subunit. (g) In this hypothetical model, two 60mers bind to a CPB dimer, creating a steric clash (arrowed brackets) between the N-terminal regions of the two molecules. Therefore, this hypothetical tetrameric complex is not expected to exist in vivo. (h) 293T cells transfected with FLAG-Plk4 were incubated with control beads or bead-immobilized Cep192 38mer (201–238) or Cep192 29mer (201–229) peptides. Beads were precipitated and analyzed by immunoblotting analysis. The same membrane was stained with Coomassie (CBB). Note that the Cep192 (201–238) peptide containing both the D-rich motif and the α-helix motif efficiently precipitated Plk4. Under the same conditions, however, the Cep192 (201–229) peptide lacking a part of the α-helix failed to bind to Plk4. This result helps explain how a molecule of Cep152-60mer (surface representation in green in Figure 4a) bound to one of the two CPB subunits can effectively interfere with the α-helix motif-dependent interaction between the 58mer and the other subunit of the CPB dimer and prevent the 58mer from binding to this site.
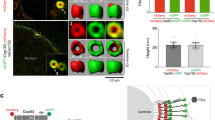
Supplementary Figure 6 Cep192 and Cep152 form two independent ring structures, and overexpressed Plk4 localizes to the inner Cep192 ring even in the presence of the Cep152 ring.
(a) U2OS cells were silenced for control Luciferase (GL siRNA), CEP192 (CEP192 siRNA), CEP152 (CEP152 siRNA), or CEP63 (CEP63 siRNA) for 48 h. The resulting cells were harvested and subjected to immunoblotting analysis. Asterisk, cross-reacting protein; CBB, Coomassie-stained membrane. (b,c) The cells in (a) were immunostained with Alexa 647 (magenta)-conjugated anti-Cep192 N-terminal (N), Alexa 488 (green)-conjugated anti-Cep152 middle region (M), and anti-Cep63 (red) antibodies (b). The relative intensities of Cep192, Cep152, and Cep63 were quantified from three independent experiments (≥30 cells/cell line/experiment) (c). Scale bars in (b), 0.2 μm; error bars in (c), s.d. (d,e) hTERT-RPE cells infected with lentivirus expressing either control vector (d) or Flag-Plk4 (e) for 12 h were continuously cultured under serum starvation conditions for 36 h. The resulting cells were released into fresh medium for 3 h, fixed, and immunostained with anti-Plk4 (pseudo-colored in yellow), Alexa 647 (magenta)-conjugated anti-Cep192 (N) and Alexa 488 (green)-conjugated anti-Cep152 (M) antibodies. The samples were then subjected to 3D-SIM microscopic analysis as described in Figure 1. Experiments were performed in triplicates. Three representative images of each control vector (d)- and Flag-Plk4 (e)-expressing cell are shown. Scale bars, 0.5 μm. Two-sided double arrows with dotted lines indicate the length of the Plk4 signals along the longitudinal axes of the centrioles. Intensity line profiles are shown in (d,e, right). For all control vector-expressing cells (≥50 centrosomes/experiment), the Plk4 signal mirrored the Cep152 signal and was not detectable in the area of the Cep192 ring (d). In contrast, cells overexpressing Flag-Plk4 exhibited greatly increased Plk4 signal at the Cep192-localized, inner regions of each cross-sectioned centrosome {upward pointing arrows in (e)}. In addition to the Plk4 signal present at the outskirts of the Cep152 rings, 137 out of 154 randomly selected centrosomes (~89%) displayed the inner Cep192-associated Plk4 signal, which was at least half the intensity of the main Plk4 peak at the outer Cep152 rings (i.e., ≥0.5 of the relative intensity shown in the graphs). Moreover, the Plk4 signal level at the inner centrosomal region was closely correlated with the Cep192 signal level, strongly suggesting that overexpressed Plk4 is recruited to the Cep192 ring structure. Consistent with this finding, Plk4 overexpression appeared to yield wider Plk4 fluorescent ring morphologies than control vector expression {compare the Plk4 ring thickness between (d) and (e)}. Rel. intensity, relative intensity. (f,g) Analysis of the images obtained in (d,e) revealed that the outer diameters of the Cep192, Cep152 and Plk4 rings in (f) were similar to those observed with U2OS cells in Figure 1. For the centrosomes with longitudinally placed centrioles, the lengths of Cep192, Cep152 and Plk4 signals along the longitudinal axes of the centrioles (see cartoon below the graph) and the ratios of their signal lengths (g) were calculated. Note that, while the length of endogenous Plk4 (~264 ± 26 nm) was similar to that of Cep152 (~259 ± 28 nm) and was about 50% of the length of the Cep192 (~525 ± 25 nm) in control cells, overexpressed Plk4 was detected along the entire length of Cep192-decorated centrioles. Error bars, s.d. from three independent experiments (≥50 centrosomes/cell line/experiment).
Supplementary Figure 7 The Cep152–Plk4 complex, but not the Cep192–Plk4 complex, binds to Cep135 without altering Plk4 kinase activity.
(a) Immunoprecipitation of Cep135 from transfected 293T cells coprecipitated Cep152, but not Cep192 (compare lane 15 with lane 11). A significant level of Plk4 was also present in the Cep135 immunoprecipitates (lane 16). HA, HA-tagged Plk4; FL, FLAG–tagged Plk4; asterisk, a signal stemming from the second anti-HA blot. “–“ indicates control vector transfection. (b) Anti-FLAG immunoprecipitates prepared from transfected 293T cells (see the Methods) were subjected to immunocomplex kinase assays using His6-Cep192 (201–310) as an in vitro substrate. The resulting samples were analyzed by both autoradiography and immunoblotting analysis with an anti-Plk4 p-S305 antibody. The level of the p-S305 epitope serves as a marker for Plk4 activity 7. “–“ indicates control vector-transfected cells. Note that Plk4 WT, but not the kinase-inactive K41M mutant (KM), phosphorylated coimmunoprecipitated Cep192 and Cep152 efficiently (compare lanes 3 and 5 with lanes 4 and 6), suggesting that Cep192 and Cep152 are Plk4 substrates in vivo. Arrowheads indicate the respective proteins. Numbers in the M lane, molecular weights (kDa) for the size markers.
Supplementary Figure 8 Uncropped immunoblot images for the main figures and antibody validation results.
(a) Related to Figure 1c, (b) Related to Figure 2a, (c) Related to Figure 2b, (d) Related to Figure 3c, (e) Related to Figure 3d, (f) Related to Figure 3e, (g) Related to Supplementary Figure 1 that used an anti-Cep192 C (2240–2538) antibody, and (h) Related to Supplementary Figure 7 that used an anti-Plk4 p-S305 antibody.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1 and 2, and Supplementary Note (PDF 16819 kb)
Rights and permissions
About this article
Cite this article
Park, SY., Park, JE., Kim, TS. et al. Molecular basis for unidirectional scaffold switching of human Plk4 in centriole biogenesis. Nat Struct Mol Biol 21, 696–703 (2014). https://doi.org/10.1038/nsmb.2846
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2846
This article is cited by
-
Architectural basis for cylindrical self-assembly governing Plk4-mediated centriole duplication in human cells
Communications Biology (2023)
-
TRIM37 controls cancer-specific vulnerability to PLK4 inhibition
Nature (2020)
-
Molecular architecture of a cylindrical self-assembly at human centrosomes
Nature Communications (2019)
-
Phase separation of Polo-like kinase 4 by autoactivation and clustering drives centriole biogenesis
Nature Communications (2019)
-
A NCAPG2-Derived Phosphopeptide Selectively Binds to the Polo-Box Domain of PLK1 and Inhibits Cancer Cell Proliferation
International Journal of Peptide Research and Therapeutics (2019)