Abstract
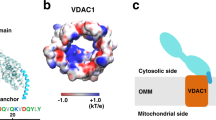
The voltage-dependent anion channel (VDAC) mediates the flow of metabolites and ions across the outer mitochondrial membrane of all eukaryotic cells. The open channel passes millions of ATP molecules per second, whereas the closed state exhibits no detectable ATP flux. High-resolution structures of VDAC1 revealed a 19-stranded β-barrel with an α-helix partially occupying the central pore. To understand ATP permeation through VDAC, we solved the crystal structure of mouse VDAC1 (mVDAC1) in the presence of ATP, revealing a low-affinity binding site. Guided by these coordinates, we initiated hundreds of molecular dynamics simulations to construct a Markov state model of ATP permeation. These simulations indicate that ATP flows through VDAC through multiple pathways, in agreement with our structural data and experimentally determined physiological rates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Törnroth-Horsefield, S. & Neutze, R. Opening and closing the metabolite gate. Proc. Natl. Acad. Sci. USA 105, 19565–19566 (2008).
Rostovtseva, T.K. et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 105, 18746–18751 (2008).
Schwarzer, C., Barnikol-Watanabe, S., Thinnes, F.P. & Hilschmann, N. Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int. J. Biochem. Cell Biol. 34, 1059–1070 (2002).
Shimizu, S., Narita, M. & Tsujimoto, Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999).
Min, C.K. et al. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 447, 371–379 (2012).
Geisler, S. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131 (2010).
Messina, A., Reina, S., Guarino, F. & De Pinto, V. VDAC isoforms in mammals. Biochim. Biophys. Acta 1818, 1466–1476 (2012).
Colombini, M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 256–257, 107–115 (2004).
Hiller, S. et al. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321, 1206–1210 (2008).
Ujwal, R. et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. USA 105, 17742–17747 (2008).
Bayrhuber, M. et al. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA 105, 15370–15375 (2008).
Rostovtseva, T. & Colombini, M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys. J. 72, 1954–1962 (1997).
Rostovtseva, T. & Colombini, M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J. Biol. Chem. 271, 28006–28008 (1996).
Báthori, G., Csordas, G., Garcia-Perez, C., Davies, E. & Hajnoczky, G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 281, 17347–17358 (2006).
Colombini, M. Voltage gating in the mitochondrial channel, VDAC. J. Membr. Biol. 111, 103–111 (1989).
Choudhary, O.P. et al. The electrostatics of VDAC: implications for selectivity and gating. J. Mol. Biol. 396, 580–592 (2010).
Rui, H., Lee, K.I., Pastor, R.W. & Im, W. Molecular dynamics studies of ion permeation in VDAC. Biophys. J. 100, 602–610 (2011).
Colombini, M. The published 3D structure of the VDAC channel: native or not? Trends Biochem. Sci. 34, 382–389 (2009).
Song, J., Midson, C., Blachly-Dyson, E., Forte, M. & Colombini, M. The topology of VDAC as probed by biotin modification. J. Biol. Chem. 273, 24406–24413 (1998).
Hiller, S., Abramson, J., Mannella, C., Wagner, G. & Zeth, K. The 3D structures of VDAC represent a native conformation. Trends Biochem. Sci. 35, 514–521 (2010).
Forte, M., Guy, H.R. & Mannella, C.A. Molecular genetics of the VDAC ion channel: structural model and sequence analysis. J. Bioenerg. Biomembr. 19, 341–350 (1987).
Yehezkel, G., Hadad, N., Zaid, H., Sivan, S. & Shoshan-Barmatz, V. Nucleotide-binding sites in the voltage-dependent anion channel: characterization and localization. J. Biol. Chem. 281, 5938–5946 (2006).
Rostovtseva, T.K. & Bezrukov, S.M. ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys. J. 74, 2365–2373 (1998).
Rostovtseva, T.K., Komarov, A., Bezrukov, S.M. & Colombini, M. Dynamics of nucleotides in VDAC channels: structure-specific noise generation. Biophys. J. 82, 193–205 (2002).
Villinger, S. et al. Nucleotide interactions of the human voltage-dependent anion channel. J. Biol. Chem. 289, 13397–13406 (2014).
Shaw, D.E. et al. in Proc. of the Conference on High Performance Computing Networking, Storage and Analysis 1–11 (Institute of Electrical and Electronics Engineers, 2009).
Sheldon, K.L., Maldonado, E.N., Lemasters, J.J., Rostovtseva, T.K. & Bezrukov, S.M. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS ONE 6, e25539 (2011).
Tomasello, M.F., Guarino, F., Reina, S., Messina, A. & De Pinto, V. The voltage-dependent anion selective channel 1 (VDAC1) topography in the mitochondrial outer membrane as detected in intact cell. PLoS ONE 8, e81522 (2013).
Colombini, M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta 1818, 1457–1465 (2012).
Singhal, N., Snow, C.D. & Pande, V.S. Using path sampling to build better Markovian state models: predicting the folding rate and mechanism of a tryptophan zipper beta hairpin. J. Chem. Phys. 121, 415–425 (2004).
Chodera, J.D., Singhal, N., Pande, V.S., Dill, K.A. & Swope, W.C. Automatic discovery of metastable states for the construction of Markov models of macromolecular conformational dynamics. J. Chem. Phys. 126, 155101 (2007).
Beauchamp, K.A. et al. MSMBuilder2: modeling conformational dynamics at the picosecond to millisecond scale. J. Chem. Theory Comput. 7, 3412–3419 (2011).
Elston, T., Wang, H. & Oster, G. Energy transduction in ATP synthase. Nature 391, 510–513 (1998).
Reimann, P., Schmid, G.J. & Hanggi, P. Universal equivalence of mean first-passage time and Kramers rate. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 60, R1–R4 (1999).
Efron, B. & Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1, 54–75 (1986).
Berezhkovskii, A., Hummer, G. & Szabo, A. Reactive flux and folding pathways in network models of coarse-grained protein dynamics. J. Chem. Phys. 130, 205102 (2009).
Metzner, P., Schutte, C. & Vanden-Eijnden, E. Transition path theory for Markov jump processes. Multiscale Model, Simul. 7, 1192–1219 (2009).
Noé, F., Schutte, C., Vanden-Eijnden, E., Reich, L. & Weikl, T.R. Constructing the equilibrium ensemble of folding pathways from short off-equilibrium simulations. Proc. Natl. Acad. Sci. USA 106, 19011–19016 (2009).
Noskov, S.Y., Rostovtseva, T.K. & Bezrukov, S.M. ATP transport through VDAC and the VDAC-tubulin complex probed by equilibrium and nonequilibrium MD simulations. Biochemistry 52, 9246–9256 (2013).
Guo, X.W. et al. Molecular design of the voltage-dependent, anion-selective channel in the mitochondrial outer membrane. J. Struct. Biol. 114, 41–59 (1995).
Koppel, D.A. et al. Bacterial expression and characterization of the mitochondrial outer membrane channel: effects of N-terminal modifications. J. Biol. Chem. 273, 13794–13800 (1998).
Colombini, M., Blachly-Dyson, E. & Forte, M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels 4, 169–202 (1996).
Duan, Y. et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 24, 1999–2012 (2003).
Cerutti, D.S., Rice, J.E., Swope, W.C. & Case, D.A. Derivation of fixed partial charges for amino acids accommodating a specific water model and implicit polarization. J. Phys. Chem. B 117, 2328–2338 (2013).
Debiec, K.T., Gronenborn, A.M. & Chong, L.T. Evaluating the strength of salt bridges: a comparison of current biomolecular force fields. J. Phys. Chem. B 10.1021/jp500958r (5 April 2014).
Jensen, M.Ø. et al. Mechanism of voltage gating in potassium channels. Science 336, 229–233 (2012).
Rostovtseva, T.K., Komarov, A., Bezrukov, S.M. & Colombini, M. VDAC channels differentiate between natural metabolites and synthetic molecules. J. Membr. Biol. 187, 147–156 (2002).
Storer, A.C. & Cornish-Bowden, A. Concentration of MgATP2− and other ions in solution: calculation of the true concentrations of species present in mixtures of associating ions. Biochem. J. 159, 1–5 (1976).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Ursby, T. & Bourgeois, D. Improved estimation of structure-factor difference amplitudes from poorly accurate data. Acta Crystallogr. A 53, 564–575 (1997).
Colletier, J.P. et al. Use of a 'caged' analogue to study the traffic of choline within acetylcholinesterase by kinetic crystallography. Acta Crystallogr. D Biol. Crystallogr. 63, 1115–1128 (2007).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Cohn, M. & Hughes, T.R. Jr. Nuclear magnetic resonance spectra of adenosine di- and triphosphate: II. Effect of complexing with divalent metal ions. J. Biol. Chem. 237, 176–181 (1962).
Liao, J.C., Sun, S., Chandler, D. & Oster, G. The conformational states of Mg.ATP in water. Eur. Biophys. J. 33, 29–37 (2004).
Jo, S., Kim, T. & Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2, e880 (2007).
MacKerell, A.D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
MacKerell, A.D. Jr., Feig, M. & Brooks, C.L. III. Improved treatment of the protein backbone in empirical force fields. J. Am. Chem. Soc. 126, 698–699 (2004).
Klauda, J.B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Foloppe, N. & MacKerell, A.D. Jr. All-atom empirical force field for nucleic acids: I. Parameter optimization based on small molecule and condensed phase macromolecular target data. J. Comput. Chem. 21, 86–104 (2000).
Pavelites, J.J., Gao, J., Bash, P.A. & Mackerell, A.D. A molecular mechanics force field for NAD+ NADH, and the pyrophosphate groups of nucleotides. J. Comput. Chem. 18, 221–239 (1997).
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. & Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Shaw, D.E. et al. Anton, a special-purpose machine for molecular dynamics simulation. Commun. ACM 51, 91–97 (2008).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Ryckaert, J.P., Ciccotti, G. & Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Bowers, K.J. et al. in Proc. of the 2006 ACM/IEEE Conference on Supercomputing 43–43 (Institute of Electrical and Electronics Engineers, 2006).
Martyna, G.J., Tobias, D.J. & Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Shan, Y., Klepeis, J.L., Eastwood, M.P., Dror, R.O. & Shaw, D.E. Gaussian split Ewald: a fast Ewald mesh method for molecular simulation. J. Chem. Phys. 122, 54101 (2005).
Gumbart, J., Khalili-Araghi, F., Sotomayor, M. & Roux, B. Constant electric field simulations of the membrane potential illustrated with simple systems. Biochim. Biophys. Acta 1818, 294–302 (2012).
Ivezic, Z., Connolly, A., VanderPlas, J. & Gray, A. Statistics, Data Mining, and Machine Learning in Astronomy (Princeton University Press, 2013).
Hille, B. Ion channels of excitable membranes (Sinauer, Sunderland, Massachusetts, USA, 2001).
Basconi, J.E. & Shirts, M.R. Effects of temperature control algorithms on transport properties and kinetics in molecular dynamics simulations. J. Chem. Theory Comput. 9, 2887–2899 (2013).
Acknowledgements
This work was supported by US National Science Foundation CAREER award MCB0845286 (M.G.) and US National Institutes of Health grants GM089740 (M.G.), T32-DK061296 (J.L.A.) and GM078844 (J.A.). O.P.C. was supported by an Andrew Mellon Predoctoral Fellowship from the University of Pittsburgh. J.-P.C. was supported by a Young International Fellowship from the Chinese Academy of Sciences. Simulations at the Texas Advanced Supercomputing Center were supported by grant MCB080011 (M.G.). The Anton special-purpose supercomputer was provided by the National Resource for Biomedical Supercomputing, the Pittsburgh Supercomputing Center and the Biomedical Technology Research Center for Multiscale Modeling of Biological Systems through grant P41GM103712-S1 from the US National Institutes of Health, and simulations on Anton were supported by grant PSCA00015P (M.G.). The Anton machine was generously made available by D.E. Shaw Research. We thank J.M. Rosenberg, K. Callenberg, F.V. Marcoline, Y. Sheng, H.Y. Wang and A. Vartanian for helpful discussions and R. Ujwal for contributions at the early stages of this work. We dedicate this work to the memory of Armand Vartanian, a colleague and friend.
Author information
Authors and Affiliations
Contributions
A.P., J.-P.C. and J.A. collected and analyzed the diffraction data. O.P.C., J.L.A. and M.G. designed, conducted and analyzed the MD simulations. The manuscript was prepared by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Electron density maps of the mVDAC1–ATP complex.
(a) A FoATP-FoNative difference Fourier map calculated between mVDAC1 ATP co-crystal and an apo crystal contoured at 3.1 σ displayed on the refined complex coordinates. (b) A solvent annealed omit map for the ATP molecule contoured at 3.3 σ. (c) A 2Fo-Fc electron density map of the refined model contoured at 1 σ. Color scheme is the same as in Fig. 1 of the main text. See Supporting Online Material for details.
Supplementary Figure 2 ATP fails to permeate mVDAC1 on the microsecond timescale.
(a,c) The z-position of the COM of the triphosphate tail and adenine ring are plotted versus time for two simulations run with either a 0 or 50 mV electric field. The channel was centered at z = 0 and aligned along the z-axis. (b,d) The same trajectories, projected onto the x-y plane. For the simulation performed with a 50 mV field, the ATP was initiated in the upper bath; the simulation at 0 mV was initiated in the pore. Despite their different initial positions within the simulation box and applied voltage, the ATP in both simulations bind to the N-terminal helix and sample a similar set of conformations. The phosphate tail remains largely immobile over the full length of the simulation, whereas the adenine ring interacts with a number of moieties within the channel.
Supplementary Figure 3 Implied timescales for the MSM.
The 50 slowest implied timescales are plotted on a log scale for a range of lag times, τ. Implied timescales were computed using Eq. 7, and the transition probability matrix obtained from the full 40 μs dataset coupled to the continuum bath as described in Supplementary Note. The lag time chosen for all analysis in the main text was 5 ns, since the timescales plateau at this point indicating that the model is Markovian.
Supplementary Figure 4 Initial placement and ATP coverage of the channel for the MSM construction.
(a) Blue spheres represent the β-phosphate atom of all 453 ATP configurations used to initiate MD simulations. The mVDAC1 channel is represented with the β-barrel in cyan and the N-terminal helix in red. The majority of simulations start with ATP in the pore domain. Membrane boundaries are indicated by black bars, but lipid and water are not shown for clarity. (b) Blue dots represent the β-phosphate of ATP plotted every 20 ps from all MD simulations initiated in panel a. The aggregate simulation time was 40 μs, and there are 10,000,000 ATP configurations represented. The densest regions are in the channel around the helix indicating that configurations in the pore domain were highly sampled. (c) Extent of ATP motion during each individual simulation. The starting z position (blue dot) and range of motion (green bar) of the COM of the ATP for each of the 453 MD simulations. The ATP undergoes 10–20 Å movements in many simulations, but never crosses the entire channel.
Supplementary Figure 5 States in the MSM are well equilibrated.
Each state in the MSM was separately analyzed for internal barriers. To do this, all configurations in each state were isolated and clustered into two substates using the k-centers k-mediods algorithm. The transition probability matrix at a 5 ns lag time was constructed, and the second eigenvalue was used to identify the relaxation time between the substates according to Eq. 7. Of the 210 states in the full MSM, 187 states (89%) relaxed faster than the 5 ns lag time used to construct the full MSM (blue circles), while 23 states (11%) relaxed much slower or failed to relax at all (red circles). Dropping these later states from the MFPT analysis had little effect on the results (data not shown).
Supplementary Figure 6 Error analysis of MFPT calculations.
(a,c) Distributions of the MFPT of ATP permeation from the cytoplasm to the IMS, and IMS to the cytoplasm, respectively, were calculated from 1000 bootstrap samples of the simulation trajectory set. (b,d) The sorted MFPTs calculated from each bootstrap sample. The mean MFPT of the distributions in a and c are indicated by a black horizontal line, while the bounds of the 95% confidence intervals are shown as dashed lines. Varying the cluster radii and lag time used to generate the MSM illustrates the sensitivity of the model to these parameters. (e) MFPTs as a function of cluster radius size for the original data set of trajectories calculated at a fixed lag time of 5 ns. (f) MFPTs as a function of lag time for the original data set of trajectories calculated at a fixed cluster radius size of 6.5 Å.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary Note (PDF 3745 kb)
Animation of the longest 4.8-μs simulation from Anton in the absence of a membrane potential.
ATP entered the channel and contacted the N-terminal α-helix during equilibration. The phosphate tail remained in contact with basic residues on the N-terminal helix except for the first few frames. The adenosine ring aggressively explored different regions of the channel including residues on the barrel wall opposite the helix. This simulation corresponds to data in Supplementary Fig. 2a,b. The left panel is the cytoplasmic view, while the right view is from the membrane. The right panel is oriented with the IMS at the bottom and the cytoplasm at the top. This orientation is used for Supplementary Movies 2, 3, 4, 5. Strands were clipped from the channel in the right panel to reveal the ATP. Mg2+ is the gold sphere adjacent to the phosphate tail. The coloring scheme for all movies is the same as images in the main text. Water and lipids were removed for clarity. (MPG 8630 kb)
Rate-limiting step along the highest-probability permeation pathway, path 1.
The rate limiting step along paths 1–4 is ATP detachment from the helix. For path 1, the phosphate tail initially interacted with just Arg15 on the helix, followed by temporary interactions with Arg15 and Arg218 on the barrel wall near the cytoplasmic mouth of the channel. Next, the interaction with Arg15 was broken, and ATP moved toward the cytosol. Supplementary Movies 2, 3, 4, 5 are continuous, representative simulations selected from the 453 simulations used for MSM construction. The frames in Supplementary Movies 2, 3, 4, 5 are spaced 200 ps apart. (MPG 5550 kb)
Rate-limiting step along paths 2–4.
ATP dissociated from the N-terminal α-helix and moved to residues Lys113 and Lys115 on the β-barrel wall. At the beginning of this movie, the phosphate tail was more closely associated with Arg15 on the helix, but it gradually broke this connection and fully interacted with Lys113 and Lys115. At this point, ATP is stretched across the pore because the adenosine ring continues to interact with the N-terminal helix. Finally, the ring broke free, and ATP moved to the barrel wall. (MPG 5537 kb)
ATP entry to the channel from the IMS.
ATP passed to Lys12 and Lys20 on the N-terminal α-helix via Lys174 on the β-barrel wall. This entry mechanism is common for paths 1–4. (MPG 6125 kb)
ATP exit to the cytosol along path 4.
ATP exited the channel along path 4 by breaking its interaction with Lys109 and diffusing into the cytoplasm. (MPG 3641 kb)
Permeation of ATP through mVDAC1 (path 1).
A complete depiction of permeation along the highest flux pathway, path 1. ATP was sequentially moved through a series of states 1, 2, 3,... N determined from the TPT analysis. Each state was defined by its generator, and the configurations from generator i to i+1 were constructed as follows. The RMSD of all snapshots in states i and i+1 were calculated with respect to the generator of state i+1. Next, we removed all snapshots that had a COM with a z-position less the z value for generator i or greater than z-position of generator i+1. We sorted the remaining snapshots in decreasing RMSD, with initial configurations closest to i and final configurations closest to i+1. This was repeated for all transitions from state 1 to state N. For capture from the IMS, we identified a single trajectory starting in bulk solution that arrived at state 1, and likewise for release to the cytoplasm, we identified a continuous trajectory starting in state N that released to the cytoplasm. The full set of ordered snapshots were then averaged in 200 ps blocks to make the final movie. ATP entered the channel from the IMS through interactions with the N-terminal methionine and Lys174 on the barrel wall. ATP subsequently moved to the center of the N-terminal helix and made contact with several basic residues. After dissociating from the helix, ATP interacted with Arg218 before exiting to the cytosol. (MPG 14026 kb)
Permeation of ATP through mVDAC1 (path 2).
A complete ATP permeation event along a flux pathway from the path 2 class of transitions. The method of movie construction is identical to the method described for Supplementary Movie 6. Initially, ATP transitioned from interacting with residue Lys174 and the N-terminal methionine to the basic residues on the N-terminal helix. The ATP then moved across the channel, where it interacted with residues Lys113 and Lys115 before transitioning to Lys161 and Arg163 at the outer mouth of the channel. Finally, ATP was released and entered the cytosol. (MPG 16317 kb)
Rights and permissions
About this article
Cite this article
Choudhary, O., Paz, A., Adelman, J. et al. Structure-guided simulations illuminate the mechanism of ATP transport through VDAC1. Nat Struct Mol Biol 21, 626–632 (2014). https://doi.org/10.1038/nsmb.2841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2841
This article is cited by
-
Mechanisms of membrane protein crystallization in ‘bicelles’
Scientific Reports (2022)
-
Mitochondrial sorting and assembly machinery operates by β-barrel switching
Nature (2021)
-
Temperature-dependent kinetic pathways of heterogeneous ice nucleation competing between classical and non-classical nucleation
Nature Communications (2021)
-
Molecular mechanism of thiamine pyrophosphate import into mitochondria: a molecular simulation study
Journal of Computer-Aided Molecular Design (2021)
-
Tuning mitochondrial structure and function to criticality by fluctuation-driven mechanotransduction
Scientific Reports (2020)