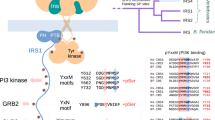
Abstract
The biological function of the PTEN tumor suppressor is mainly attributed to its lipid phosphatase activity. This study demonstrates that mammalian PTEN is a protein tyrosine phosphatase that selectively dephosphorylates insulin receptor substrate-1 (IRS1), a mediator of insulin and IGF signals. IGF signaling was defective in cells lacking NEDD4, a PTEN ubiquitin ligase, whereas AKT activation triggered by EGF or serum was unimpaired. Defective IGF signaling caused by NEDD4 deletion, including phosphorylation of IRS1 and AKT, was rescued by PTEN ablation. We demonstrate the nature of PTEN as an IRS1 phosphatase by direct biochemical analysis and cellular reconstitution, showing that NEDD4 supports insulin-mediated glucose metabolism and is required for the proliferation of IGF1 receptor–dependent but not EGF receptor–dependent tumor cells. Thus, PTEN is a protein phosphatase for IRS1, and its antagonism by NEDD4 promotes signaling by IGF and insulin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Steck, P.A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997).
Song, M.S., Salmena, L. & Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012).
Parsons, R. & Simpson, L. PTEN and cancer. Methods Mol. Biol. 222, 147–166 (2003).
Engelman, J.A., Luo, J. & Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 (1998).
Lu, Y. et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 18, 7034–7045 (1999).
Maehama, T. & Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Li, D.M. & Sun, H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor β. Cancer Res. 57, 2124–2129 (1997).
Lee, J.O. et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Myers, M.P. et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl. Acad. Sci. USA 94, 9052–9057 (1997).
Maier, D. et al. The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 59, 5479–5482 (1999).
Tamura, M., Gu, J., Takino, T. & Yamada, K.M. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 59, 442–449 (1999).
Tibarewal, P. et al. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci. Signal. 5, ra18 (2012).
Zhang, X.C., Piccini, A., Myers, M.P., Van Aelst, L. & Tonks, N.K. Functional analysis of the protein phosphatase activity of PTEN. Biochem. J. 444, 457–464 (2012).
Wang, X. & Jiang, X. Post-translational regulation of PTEN. Oncogene 27, 5454–5463 (2008).
Keniry, M. & Parsons, R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 27, 5477–5485 (2008).
Wang, X. et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Trotman, L.C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Fouladkou, F. et al. The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl. Acad. Sci. USA 105, 8585–8590 (2008).
Drinjakovic, J. et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 65, 341–357 (2010).
Christie, K.J., Martinez, J.A. & Zochodne, D.W. Disruption of E3 ligase NEDD4 in peripheral neurons interrupts axon outgrowth: linkage to PTEN. Mol. Cell. Neurosci. 50, 179–192 (2012).
Howitt, J. et al. Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia. J. Cell Biol. 196, 29–36 (2012).
Guo, H. et al. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Reports 1, 472–482 (2012).
Yim, E.K. et al. Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell 15, 304–314 (2009).
Mund, T. & Pelham, H.R. Regulation of PTEN/Akt and MAP kinase signaling pathways by the ubiquitin ligase activators Ndfip1 and Ndfip2. Proc. Natl. Acad. Sci. USA 107, 11429–11434 (2010).
Cao, X.R. et al. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci. Signal. 1, ra5 (2008).
Chen, W.S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Cho, H., Thorvaldsen, J.L., Chu, Q., Feng, F. & Birnbaum, M.J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 (2001).
Liu, J.P., Baker, J., Perkins, A.S., Robertson, E.J. & Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993).
Taniguchi, C.M., Emanuelli, B. & Kahn, C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Maccario, H., Perera, N.M., Gray, A., Downes, C.P. & Leslie, N.R. Ubiquitination of PTEN (phosphatase and tensin homolog) inhibits phosphatase activity and is enhanced by membrane targeting and hyperosmotic stress. J. Biol. Chem. 285, 12620–12628 (2010).
Davidson, L. et al. Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29, 687–697 (2010).
Lackey, J. et al. Loss of PTEN selectively desensitizes upstream IGF1 and insulin signaling. Oncogene 26, 7132–7142 (2007).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Goldstein, B.J., Bittner-Kowalczyk, A., White, M.F. & Harbeck, M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B: possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275, 4283–4289 (2000).
Galic, S. et al. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 25, 819–829 (2005).
Tiganis, T. PTP1B and TCPTP: nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 280, 445–458 (2013).
Casaletto, J.B. & McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 12, 387–400 (2012).
Anindya, R., Aygün, O. & Svejstrup, J.Q. Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol. Cell 28, 386–397 (2007).
Yang, B. et al. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat. Immunol. 9, 1356–1363 (2008).
Lin, Q. et al. HECT E3 ubiquitin ligase Nedd4-1 ubiquitinates ACK and regulates epidermal growth factor (EGF)-induced degradation of EGF receptor and ACK. Mol. Cell. Biol. 30, 1541–1554 (2010).
Persaud, A. et al. Comparison of substrate specificity of the ubiquitin ligases Nedd4 and Nedd4-2 using proteome arrays. Mol. Syst. Biol. 5, 333 (2009).
Acknowledgements
We thank B. Yang (University of Iowa) for providing NEDD4−/− MEFs and paired NEDD4+/+ MEFs, J. Lee for excellent technical support and N. Pavletich for discussing the structural basis of the defects of various PTEN mutants. We also thank members of Jiang laboratory and D. Marks for discussing and reading the manuscript. This work is supported by an American Cancer Society scholar award (to X.J.) and by funding from Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research of the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (to X.J.) and the Geoffrey Beene Cancer Research foundation (to X.J., N.R. and S.C.).
Author information
Authors and Affiliations
Contributions
Y.S., N.R. and X.J. designed the study; Y.S., J.W. and J.C. performed the experiments; Y.S., S.C., N.R. and X.J. wrote the paper; and all authors were involved in data analysis and interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 NEDD4 specifically regulates IGF but not EGF or serum signaling.
(a) Western blotting analysis of (AKT phosphorylation) upon different stimuli. NEDD4+/+ and NEDD4-/- MEFs were serum starved for 3 hrs, followed by 5 min stimulation with 10% serum, IGF1 (50 ng/ml), or 50 ng/ml, 100 ng/ml IGF2. β-actin is used as a loading/sample preparation control. (b) Western blotting analysis similar as in (a), with different amounts of EGF; EGFR phosphorylation was also assessed. γ-tubulin is used as loading control. (c) Western blots analyses of time course of the effect of NEDD4 elimination on insulin signaling. MEFs harboring Dox-inducible NEDD4 shRNA construct were treated with or without 1 μg/ml Dox for 3 days, serum-starved for 3 hrs, and then stimulated for the indicated amount of time with insulin (100 ng/ml).
Supplementary Figure 2 Characterization of recombinant PTEN protein in lipid and protein phosphatase assays.
(a) WT PTEN but not the CS or GE mutant dephosphorylates PIP3. The assay was performed as described in Methods. 40 μM PIP3 and 0.1 μM PTEN (WT), 10 μM PTEN (CS) and 10 μM PTEN (GE) proteins were used in the assay. WT: wild-type; CS: C124S mutant; GE: G129E mutant. (b) Coomassie blue gel showing purified recombinant PTEN proteins. YL: Y138L mutant. (c) WT-PTEN or GE but not YL or CS mutant can dephosphorylate IRS1. Immunoprecipitated IRS1 was incubated with recombinant PTEN (WT, YL GE or CS mutant) protein (0.6 μM) for 1 hr at 30 °C. (d) Comparing PTEN (WT or mutants) activity in lipid phosphatase assay. The assay was performed similar as in (a), with 40 μM PIP3 and PTEN (WT/YL/CS/GE) proteins of indicated concentrations. (e) Comparison of PTP1B and PTEN activity in the in vitro protein phosphatase assays. The assay was performed similar as in (c), with substrates (IGF1R and IRS1) and enzymes (PTP1B and PTEN) as indicated, and quantitated by densitometry.
Supplementary Figure 3 WT and GE mutant but not CS mutant of PTEN can dephosphorylate IRS1 in cells.
(a)Validation of PTEN shRNA MEFs reconstituted with equal amount of GFP-S-tagged PTEN (WT/CS/GE). (b-e) The experiments were performed the same as that in Figure 4 except that both p-IRS1 (Y608) and p-IRS1 (Y989) were monitored here. (f) PI3K inhibitors block IGF1-induced phosphorylation of AKT but not that of IGF1R or IRS1. WT MEFs were pretreated for 1 hr with either 2 μM BYL-719 or 1 μM GDC0941, followed by 50 ng/ml IGF1 for 10 min.
Supplementary Figure 4 PTEN and AKT regulate IGF1R expression via feedback mechanism.
(a) PTEN-/- MEFs express lower level of IGF1R which can be increased by AKT inhibition. WT or PTEN-/- MEFs were treated with 1 μM AKTi for 12 or 24 hrs. γ-tubulin is used as loading control. (b) MEFs harboring long-term PTEN shRNA have a decreased IGF1R expression, which can be reversed by AKT inhibition. β-actin is used as loading control. (c) Expression of wild-type but not CS or GE mutant PTEN increased IGF1R expression in PTEN-/- MEFs. PTEN-/- MEFs were reconstituted with GFP or GFP-S-PTEN (WT/CS/GE), and treated with or without 1 μM AKTi for 24 hrs. (d) Stable PTEN shRNA restored IGF1-induced AKT activation in NEDD4-/- MEFs but had minimal effect on IRS1 phosphorylation. NEDD4-/- MEFs with control knockdown or stable PTEN knockdown were serum-starved, and then stimulated with 50 ng/ml IGF1 for 5 min, with or without 1 μM PI3K inhibitor
Supplementary Figure 5 NEDD4 is required for AKT activity in MCF7 but not MDA-MB-468 cells.
(a) The effect of IGF1R inhibitor, EGFR inhibitor on AKT activity in MCF7 and MDA-MB-468 cells. MCF7 and MDA-MB-468 cells were treated with either 5 μM IGF1Ri or 5 μM EGFRi for 3 hrs. γ-tubulin is used as loading control. (b) The effect of NEDD4 RNAi on AKT activity in MCF7 and MDA-MB-468 cells. MCF7 and MDA-MB-468 cells harboring Dox-inducible NEDD4 shRNA construct were treated with or without 1 μg/ml Dox for 3 days
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 571 kb)
Supplementary Data Set 1
Uncropped images of gels (PDF 2222 kb)
Rights and permissions
About this article
Cite this article
Shi, Y., Wang, J., Chandarlapaty, S. et al. PTEN is a protein tyrosine phosphatase for IRS1. Nat Struct Mol Biol 21, 522–527 (2014). https://doi.org/10.1038/nsmb.2828
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2828
This article is cited by
-
Targeting the E3 ligase NEDD4 as a novel therapeutic strategy for IGF1 signal pathway-driven gastric cancer
Oncogene (2023)
-
Understanding Glycogen Synthase Kinase-3: A Novel Avenue for Alzheimer’s Disease
Molecular Neurobiology (2023)
-
PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation
Journal of Biomedical Science (2022)
-
The equilibrium of tumor suppression: DUBs as active regulators of PTEN
Experimental & Molecular Medicine (2022)
-
The phosphorylation and dephosphorylation switch of VCP/p97 regulates the architecture of centrosome and spindle
Cell Death & Differentiation (2022)