Abstract
The initial stage of CRISPR–Cas immunity involves the integration of foreign DNA spacer segments into the host genomic CRISPR locus. The nucleases Cas1 and Cas2 are the only proteins conserved among all CRISPR–Cas systems, yet the molecular functions of these proteins during immunity are unknown. Here we show that Cas1 and Cas2 from Escherichia coli form a stable complex that is essential for spacer acquisition and determine the 2.3-Å-resolution crystal structure of the Cas1–Cas2 complex. Mutations that perturb Cas1–Cas2 complex formation disrupt CRISPR DNA recognition and spacer acquisition in vivo. Active site mutants of Cas2, unlike those of Cas1, can still acquire new spacers, thus indicating a nonenzymatic role of Cas2 during immunity. These results reveal the universal roles of Cas1 and Cas2 and suggest a mechanism by which Cas1–Cas2 complexes specify sites of CRISPR spacer integration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sorek, R., Lawrence, C.M. & Wiedenheft, B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu. Rev. Biochem. 82, 237–266 (2013).
Mojica, F.J., Diez-Villasenor, C., Garcia-Martinez, J. & Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 (2005).
Bolotin, A., Quinquis, B., Sorokin, A. & Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151, 2551–2561 (2005).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Yosef, I., Goren, M.G. & Qimron, U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 40, 5569–5576 (2012).
Swarts, D.C., Mosterd, C., van Passel, M.W. & Brouns, S.J. CRISPR interference directs strand specific spacer acquisition. PLoS ONE 7, e35888 (2012).
Datsenko, K.A. et al. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat. Commun. 3, 945 (2012).
Brouns, S.J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Carte, J., Wang, R., Li, H., Terns, R.M. & Terns, M.P. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496 (2008).
Haurwitz, R.E., Jinek, M., Wiedenheft, B., Zhou, K. & Doudna, J.A. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science 329, 1355–1358 (2010).
Deltcheva, E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011).
Sashital, D.G., Jinek, M. & Doudna, J.A. An RNA-induced conformational change required for CRISPR RNA cleavage by the endoribonuclease Cse3. Nat. Struct. Mol. Biol. 18, 680–687 (2011).
Wiedenheft, B. et al. Structures of the RNA-guided surveillance complex from a bacterial immune system. Nature 477, 486–489 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Garneau, J.E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Jore, M.M. et al. Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nat. Struct. Mol. Biol. 18, 529–536 (2011).
Makarova, K.S. et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 9, 467–477 (2011).
Savitskaya, E., Semenova, E., Dedkov, V., Metlitskaya, A. & Severinov, K. High-throughput analysis of type I-E CRISPR/Cas spacer acquisition in E. coli. RNA Biol. 10, 716–725 (2013).
Diez-Villaseñor, C., Guzman, N.M., Almendros, C., Garcia-Martinez, J. & Mojica, F.J. CRISPR-spacer integration reporter plasmids reveal distinct genuine acquisition specificities among CRISPR-Cas I-E variants of Escherichia coli. RNA Biol. 10, 792–802 (2013).
Mojica, F.J., Diez-Villasenor, C., Garcia-Martinez, J. & Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 155, 733–740 (2009).
Sashital, D.G., Wiedenheft, B. & Doudna, J.A. Mechanism of foreign DNA selection in a bacterial adaptive immune system. Mol. Cell 46, 606–615 (2012).
Beloglazova, N. et al. A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats. J. Biol. Chem. 283, 20361–20371 (2008).
Wiedenheft, B. et al. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure 17, 904–912 (2009).
Babu, M. et al. A dual function of the CRISPR-Cas system in bacterial antivirus immunity and DNA repair. Mol. Microbiol. 79, 484–502 (2011).
Nam, K.H. et al. Double-stranded endonuclease activity in Bacillus halodurans clustered regularly interspaced short palindromic repeats (CRISPR)-associated Cas2 protein. J. Biol. Chem. 287, 35943–35952 (2012).
Kim, T.Y., Shin, M., Huynh Thi Yen, L. & Kim, J.S. Crystal structure of Cas1 from Archaeoglobus fulgidus and characterization of its nucleolytic activity. Biochem. Biophys. Res. Commun. 441, 720–725 (2013).
Diez-Villaseñor, C., Almendros, C., Garcia-Martinez, J. & Mojica, F.J. Diversity of CRISPR loci in Escherichia coli. Microbiology 156, 1351–1361 (2010).
Goren, M.G., Yosef, I., Auster, O. & Qimron, U. Experimental definition of a clustered regularly interspaced short palindromic duplicon in Escherichia coli. J. Mol. Biol. 423, 14–16 (2012).
Samai, P., Smith, P. & Shuman, S. Structure of a CRISPR-associated protein Cas2 from Desulfovibrio vulgaris. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1552–1556 (2010).
Han, D., Lehmann, K. & Krauss, G. SSO1450–a CAS1 protein from Sulfolobus solfataricus P2 with high affinity for RNA and DNA. FEBS Lett. 583, 1928–1932 (2009).
Makarova, K.S., Anantharaman, V., Aravind, L. & Koonin, E.V. Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 7, 40 (2012).
Plagens, A., Tjaden, B., Hagemann, A., Randau, L. & Hensel, R. Characterization of the CRISPR/Cas subtype I-A system of the hyperthermophilic crenarchaeon Thermoproteus tenax. J. Bacteriol. 194, 2491–2500 (2012).
Richter, C., Gristwood, T., Clulow, J.S. & Fineran, P.C. In vivo protein interactions and complex formation in the Pectobacterium atrosepticum subtype I-F CRISPR/Cas System. PLoS ONE 7, e49549 (2012).
Kranzusch, P.J., Lee, A.S., Berger, J.M. & Doudna, J.A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Reports 3, 1362–1368 (2013).
Van Duyne, G.D., Standaert, R.F., Karplus, P.A., Schreiber, S.L. & Clardy, J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 (1993).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Laue, T.M., Shah, B.D., Ridgeway, T.M. & Pelletier, S.L. Analytical Ultracentrifugation in Biochemistry and Polymer Science 90–125 (Royal Society of Chemistry, 1992).
Brown, P.H. & Schuck, P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys. J. 90, 4651–4661 (2006).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Pei, J., Kim, B.H. & Grishin, N.V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 (2008).
Waterhouse, A.M., Procter, J.B., Martin, D.M., Clamp, M. & Barton, G.J. Jalview Version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Acknowledgements
We are grateful for the input on this work provided by members of the Doudna laboratory. We thank S. Floor, A.S. Lee, H.Y. Lee, R. Wilson, R. Wu and K. Zhou for technical assistance, the 8.3.1 beamline staff at the Advanced Light Source and A. Iavarone (University of California, Berkeley) for MS. We thank D. King (Howard Hughes Medical Institute, University of California, Berkeley) for Flag and HA peptides. This project was funded by a US National Science Foundation grant to J.A.D. (no. 1244557). J.K.N. and A.V.W. are supported by US National Science Foundation Graduate Research Fellowships and J.K.N. by a University of California, Berkeley Chancellor's Fellowship. P.J.K. is supported as a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. J.N. is supported by a Long-Term Postdoctoral Fellowship from the Human Frontier Science Program Organization. J.A.D. is supported as an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.K.N. performed the protein purification, biochemical and crystallography experiments. X-ray diffraction data were collected by J.K.N., P.J.K. and J.N., and structure determination was performed by J.K.N. and P.J.K. A.V.W. assisted J.K.N. with in vivo acquisition and immunoprecipitation assays. C.W.D. performed and analyzed analytical ultracentrifugation experiments. J.K.N. and J.A.D. designed the study, analyzed all data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
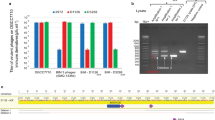
Supplementary Figure 1 In vivo acquisition with epitope-tagged Cas1 and Cas2 and in vitro reconstitution of the complex.
(a) Agarose gel of acquisition assays in BL21-AI cells overexpressing Cas1 and Cas2 with or without epitope tags. Lanes labeled ‘starter’ indicate the starter culture before inoculation into cultures in inducing (+) or non-inducing (-) conditions. The Cas1-FLAG and Cas2-HA constructs were used for the immunoprecipitation and DNA affinity precipitation experiments in this study. (b) An overlay of the c(s) distributions of Cas1 only (solid black), Cas2 only (dotted) and Cas1–Cas2 complex (solid blue). (c) AUC data table highlighting the s-values and the calculated apparent molecular weights. (d) Gel filtration chromatogram of pre-incubated, separately purified Cas1 and Cas2, as described in the Methods section. The arrow points to the expected elution peak of Cas1 dimer, based on our protein purification. (e) Coomassie-stained SDS-PAGE of fractions corresponding to the two peaks in (b). The green arrow points to Cas1 (33.7 kDa) and the orange arrow points to Cas2 (11.6 kDa).
Supplementary Figure 2 The two Cas1 dimers are structurally similar.
(a,b) Two views of the superposition of the Cas1a-b dimer (teal and blue) with the Cas1c-d dimer (gray). The root-mean-square deviation for the C-alpha backbone is 0.394. (c) The Cas1c–Cas2 interface is similar to the Cas1a–Cas2 interface, shown in Figure 3b. (d) Gel filtration chromatogram of purified Cas1 R252E using a Superdex 75 (16/60) size exclusion column.
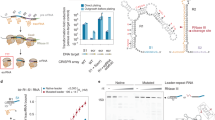
Supplementary Figure 3 Cas1–Cas2 complex formation is required for CRISPR DNA binding.
(a) A schematic of the biotinylated DNA affinity precipitation experiments conducted in this study, as further described in the Methods section. (b) Western blot of the fractions throughout the experiment using magnetic Streptavidin beads, as opposed to streptavidin-agarose resin shown in Fig. 4e. (c) A schematic of the six 125-bp, biotinylated DNA substrates with scrambled regions within the leader sequence, shown in gray. Substrate 6 has a random DNA sequence upstream of the repeat with no similarity to the leader sequence (d) Western blot to detect Cas1 levels in the elution fractions of DNA affinity precipitations using the six biotinylated DNA substrates in BL21-AI cells overexpressiong Cas1 and Cas2. (e) Elution samples of DNA affinity precipitations in lysates from BL21-AI cells overexpressing the indicated Cas1 and Cas2 mutants. The +/- annotations are results from Fig. 3.
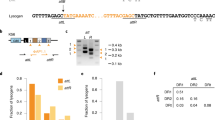
Supplementary Figure 4 Structure-based Cas1 and Cas2 alignments.
The E. coli Cas1 (a) or Cas2 (b) protein was aligned to its respective homologs for which crystal structures are available, as described in the Methods section. The number annotations at the C-termini refer to the last amino acid residue resolved in the structure, followed by the last residue of the full-length protein. The secondary structure cartoon is for the E. coli protein and the annotated residues (red) refer to the ones that were mutated in this study. The BLOSUM62 score conservation threshold is set to 50%, as reflected by the blue colors of the alignment. (c) Two views of a structure alignment of the Cas2 in the Cas1–Cas2 complex with all the available crystal structures of Cas2 homologs (all shown in monomers).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1 (PDF 14926 kb)
Rights and permissions
About this article
Cite this article
Nuñez, J., Kranzusch, P., Noeske, J. et al. Cas1–Cas2 complex formation mediates spacer acquisition during CRISPR–Cas adaptive immunity. Nat Struct Mol Biol 21, 528–534 (2014). https://doi.org/10.1038/nsmb.2820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2820
This article is cited by
-
Temporally resolved transcriptional recording in E. coli DNA using a Retro-Cascorder
Nature Protocols (2023)
-
Histones direct site-specific CRISPR spacer acquisition in model archaeon
Nature Microbiology (2023)
-
Structural and functional analyses of Burkholderia pseudomallei BPSL1038 reveal a Cas-2/VapD nuclease sub-family
Communications Biology (2023)
-
The Phylogenetic Study of the CRISPR-Cas System in Enterobacteriaceae
Current Microbiology (2023)
-
Recording gene expression order in DNA by CRISPR addition of retron barcodes
Nature (2022)