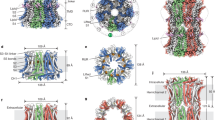
Abstract
Calmodulin (CaM) is a universal regulatory protein that communicates the presence of calcium to its molecular targets and correspondingly modulates their function. This key signaling protein is important for controlling the activity of hundreds of membrane channels and transporters. However, understanding of the structural mechanisms driving CaM regulation of full-length membrane proteins has remained elusive. In this study, we determined the pseudoatomic structure of full-length mammalian aquaporin-0 (AQP0, Bos taurus) in complex with CaM, using EM to elucidate how this signaling protein modulates water-channel function. Molecular dynamics and functional mutation studies reveal how CaM binding inhibits AQP0 water permeability by allosterically closing the cytoplasmic gate of AQP0. Our mechanistic model provides new insight, only possible in the context of the fully assembled channel, into how CaM regulates multimeric channels by facilitating cooperativity between adjacent subunits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Saimi, Y. & Ling, K.Y. Calmodulin activation of calcium-dependent sodium channels in excised membrane patches of Paramecium. Science 249, 1441–1444 (1990).
Zühlke, R.D., Pitt, G.S., Deisseroth, K., Tsien, R.W. & Reuter, H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399, 159–162 (1999).
Tan, H.L. et al. A calcium sensor in the sodium channel modulates cardiac excitability. Nature 415, 442–447 (2002).
Saimi, Y. & Kung, C. Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 64, 289–311 (2002).
Meissner, G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry 25, 244–251 (1986).
Smith, J.S., Rousseau, E. & Meissner, G. Calmodulin modulation of single sarcoplasmic reticulum Ca2+-release channels from cardiac and skeletal muscle. Circ. Res. 64, 352–359 (1989).
Zalk, R., Lehnart, S.E. & Marks, A.R. Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 76, 367–385 (2007).
Scott, K., Sun, Y., Beckingham, K. & Zuker, C.S. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell 91, 375–383 (1997).
Zhang, Z. et al. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc. Natl. Acad. Sci. USA 98, 3168–3173 (2001).
Zhu, M.X. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 451, 105–115 (2005).
Peracchia, C., Sotkis, A., Wang, X.G., Peracchia, L.L. & Persechini, A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 275, 26220–26224 (2000).
Sotkis, A. et al. Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell Commun. Adhes. 8, 277–281 (2001).
Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 1662, 61–80 (2004).
Németh-Cahalan, K.L. & Hall, J.E. pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 275, 6777–6782 (2000).
Varadaraj, K., Kumari, S., Shiels, A. & Mathias, R.T. Regulation of aquaporin water permeability in the lens. Invest. Ophthalmol. Vis. Sci. 46, 1393–1402 (2005).
Babu, Y.S. et al. Three-dimensional structure of calmodulin. Nature 315, 37–40 (1985).
Zhang, M., Tanaka, T. & Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 2, 758–767 (1995).
Kuboniwa, H. et al. Solution structure of calcium-free calmodulin. Nat. Struct. Biol. 2, 768–776 (1995).
Chou, J.J., Li, S., Klee, C.B. & Bax, A. Solution structure of Ca2+–calmodulin reveals flexible hand-like properties of its domains. Nat. Struct. Biol. 8, 990–997 (2001).
Vogel, H.J. The Merck Frosst Award Lecture 1994. Calmodulin: a versatile calcium mediator protein. Biochem. Cell Biol. 72, 357–376 (1994).
Yamniuk, A.P. & Vogel, H.J. Calmodulin's flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol. 27, 33–57 (2004).
Gonen, T. & Walz, T. The structure of aquaporins. Q. Rev. Biophys. 39, 361–396 (2006).
Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605 (2000).
de Groot, B.L., Frigato, T., Helms, V. & Grubmuller, H. The mechanism of proton exclusion in the aquaporin-1 water channel. J. Mol. Biol. 333, 279–293 (2003).
Bloemendal, H., Zweers, A., Vermorken, F., Dunia, I. & Benedetti, E.L. The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1, 91–106 (1972).
Németh-Cahalan, K.L., Kalman, K. & Hall, J.E. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J. Gen. Physiol. 123, 573–580 (2004).
Mulders, S.M. et al. Water channel properties of major intrinsic protein of lens. J. Biol. Chem. 270, 9010–9016 (1995).
Chandy, G., Zampighi, G.A., Kreman, M. & Hall, J.E. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol. 159, 29–39 (1997).
Harries, W.E., Akhavan, D., Miercke, L.J., Khademi, S. & Stroud, R.M. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc. Natl. Acad. Sci. USA 101, 14045–14050 (2004).
Gonen, T. et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638 (2005).
Bok, D., Dockstader, J. & Horwitz, J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J. Cell Biol. 92, 213–220 (1982).
Costello, M.J., McIntosh, T.J. & Robertson, J.D. Distribution of gap junctions and square array junctions in the mammalian lens. Invest. Ophthalmol. Vis. Sci. 30, 975–989 (1989).
Gonen, T., Sliz, P., Kistler, J., Cheng, Y. & Walz, T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 429, 193–197 (2004).
Girsch, S.J. & Peracchia, C. Calmodulin interacts with a C-terminus peptide from the lens membrane protein MIP26. Curr. Eye Res. 10, 839–849 (1991).
Reichow, S.L. & Gonen, T. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure 16, 1389–1398 (2008).
Schumacher, M.A., Rivard, A.F., Bachinger, H.P. & Adelman, J.P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410, 1120–1124 (2001).
Van Petegem, F., Chatelain, F.C. & Minor, D.L. Jr. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain–Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 12, 1108–1115 (2005).
Mori, M.X., Vander Kooi, C.W., Leahy, D.J. & Yue, D.T. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+. Structure 16, 607–620 (2008).
Sarhan, M.F., Tung, C.C., Van Petegem, F. & Ahern, C.A. Crystallographic basis for calcium regulation of sodium channels. Proc. Natl. Acad. Sci. USA 109, 3558–3563 (2012).
Samsó, M. & Wagenknecht, T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J. Biol. Chem. 277, 1349–1353 (2002).
Huang, X., Fruen, B., Farrington, D.T., Wagenknecht, T. & Liu, Z. Calmodulin-binding locations on the skeletal and cardiac ryanodine receptors. J. Biol. Chem. 287, 30328–30335 (2012).
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Grigorieff, N. FREALIGN: high-resolution refinement of single particle structures. J. Struct. Biol. 157, 117–125 (2007).
Pettersen, E.F. et al. UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Yap, K.L., Yuan, T., Mal, T.K., Vogel, H.J. & Ikura, M. Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J. Mol. Biol. 328, 193–204 (2003).
Fallon, J.L. et al. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2+* calmodulins. Proc. Natl. Acad. Sci. USA 106, 5135–5140 (2009).
Kim, E.Y. et al. Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization. EMBO J. 29, 3924–3938 (2010).
Smart, O.S., Neduvelil, J.G., Wang, X., Wallace, B.A. & Sansom, M.S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996).
Jensen, M.Ø. & Mouritsen, O.G. Single-channel water permeabilities of Escherichia coli aquaporins AqpZ and GlpF. Biophys. J. 90, 2270–2284 (2006).
Jensen, M.Ø. et al. Dynamic control of slow water transport by aquaporin 0: implications for hydration and junction stability in the eye lens. Proc. Natl. Acad. Sci. USA 105, 14430–14435 (2008).
Hashido, M., Ikeguchi, M. & Kidera, A. Comparative simulations of aquaporin family: AQP1, AQPZ, AQP0 and GlpF. FEBS Lett. 579, 5549–5552 (2005).
Yang, B. & Verkman, A.S. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 272, 16140–16146 (1997).
Yuan, T. & Vogel, H.J. Calcium-calmodulin-induced dimerization of the carboxyl-terminal domain from petunia glutamate decarboxylase: a novel calmodulin-peptide interaction motif. J. Biol. Chem. 273, 30328–30335 (1998).
Gut, H. et al. A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 392, 334–351 (2009).
Xia, X.M. et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395, 503–507 (1998).
Schumacher, M.A., Crum, M. & Miller, M.C. Crystal structures of apocalmodulin and an apocalmodulin/SK potassium channel gating domain complex. Structure 12, 849–860 (2004).
Wang, C., Wang, H.G., Xie, H. & Pitt, G.S. Ca2+/CaM controls Ca2+-dependent inactivation of NMDA receptors by dimerizing the NR1 C termini. J. Neurosci. 28, 1865–1870 (2008).
Shi, L.B., Skach, W.R. & Verkman, A.S. Functional independence of monomeric CHIP28 water channels revealed by expression of wild-type mutant heterodimers. J. Biol. Chem. 269, 10417–10422 (1994).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
MacKerell, A.D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Klauda, J.B., Brooks, B.R., MacKerell, A.D. Jr., Venable, R.M. & Pastor, R.W. An ab initio study on the torsional surface of alkanes and its effect on molecular simulations of alkanes and a DPPC bilayer. J. Phys. Chem. B 109, 5300–5311 (2005).
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. & Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Grubmuller, H., Heller, H., Windermuth, A. & Schulten, K. Generalized Verlet algorithm for efficient molecular dynamics simulations with long-range interactions. Mol. Simul. 6, 121–142 (1991).
Martyna, G.J., Tobiax, D.J. & Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Feller, S.E., Zhang, Y., Pastor, R.W. & Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J Mol. Graph. 14, 33–38 (1996).
Acknowledgements
The authors would like to thank M. Sarhan (Howard Hughes Medical Institute (HHMI), Janelia Farm Research Campus) for help with ITC and D. Shi (HHMI, Janelia Farm Research Campus) for help with various aspects of EM. Research in the laboratory of J.E.H. is supported by US National Institutes of Health (NIH) National Eye Institute grant EY5661 (J.E.H.). Research by D.M.C. was supported by the NIH National Library of Medicine Biomedical Informatics Research Training Program Award, no. LM007443. Research by S.L.R. was supported by the Ruth L. Kirschtein National Research Service Award from NIH. Research in the laboratory of D.J.T. is supported by NIH National Institute of Neurological Disorders–National Institute of General Medical Sciences grant GM86685 and US National Science Foundation grant CHE-0750175 (D.J.T.). M.H. is supported by a fellowship from the German Academy of Sciences Leopoldina. This work was supported in part by NIH grant R01 GM079233 (T.G.). Research in the laboratory of T.G. is funded by the HHMI (T.G.).
Author information
Authors and Affiliations
Contributions
S.L.R., D.M.C., J.E.H. and T.G. conceived of and designed the experiments for this work. All authors contributed to data analysis and preparation of the manuscript. S.L.R. performed protein purification, EM and ITC binding studies on the AQP0–CaM complexes. D.M.C., J.A.F., M.H. and D.J.T. performed setup and analysis of molecular dynamics simulations. D.M.C. and K.L.N.-C. performed oocyte permeability measurements, construction of oocyte expression constructs and analysis of oocyte permeability data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Purification of the cross-linked AQP0–CaM complex.
Schematic of crosslinking and purification of the AQP0–CaM complex (EDC/NHS – crosslinking reagents; IEX – ion exchange chromatography; SEC – size exclusion chromatography). (b and c) Separation of AQP0–CaM from unreacted AQP0 by IEX. (UV(280nm) – blue; conductivity – red). Selected fractions for all steps indicated in grey. (d) Separation of the AQP0–CaM from free CaM by SEC. (inset) Molecular weight calibration showing elution of the AQP0–CaM complex at ~180 KDa; blue dot. (e) SDS-PAGE (lane 1) CaM alone, treated with EDC/NHS resulted in two bands, corresponding to CaM ~13 KDa and the crosslinked CaM dimer (CaM)2 ~22 KDa. (lane 2) AQP0 alone, treated with EDC/NHS resulted in at least four bands, corresponding to the AQP0 monomer ~26 KDa, the crosslinked dimer (AQP0)2 ~52 KDa, trimer (AQP0)3 ~80 KDa, and tetramer (AQP0)4 ~110 KDa. (lane 3) Crude crosslinking products following addition of activated CaM to AQP0 (corresponding to step 2 in (a)). (lane 4) Flow-through from IEX #1. (lanes 5 and 6) Fractions from IEX #1 containing unreacted AQP0 and crosslinked AQP0–CaM products, respectively. (lane 7) Flow-through from IEX #2. (lanes 8 and 9) Fraction from IEX #2 containing unreacted AQP0 and crosslinked AQP0–CaM products, respectively. (lane 10) SEC peak fraction containing AQP0–CaM. Note lanes 3, 6, 9 and 10 contain two unique bands at ~39 KDa and ~65 KDa correspond to the crosslinked 1:1 AQP0–CaM complex and the 2:1 (AQP0)2–CaM denatured complexes.
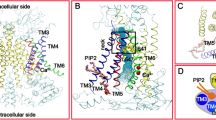
Supplementary Figure 2 Construction of the pseudoatomic model of AQP0–CaM derived by EM.
(Step 1) The crystallographic structure of AQP0 (PDB 2B6P)29 was chosen for the transmembrane domain of the complex. (Step 2) The cytoplasmic C-terminal domains (residues 223-263) of AQP0 were removed. (Step 3) The AQP0 transmembrane domain was computationally fit into the EM map using Chimera44. (Step 4) The α-helical region of the AQP0CBD (residues 227–241) was threaded onto the two anti-parallel α-helices (Helix A and Helix B) in the ptGAD–CaM structure (PDB 1NWD)45. The AQP0CBD residue L234 occupies the site equivalent to the major hydrophobic anchoring residue in the ptGAD–CaM complex (W485)45. Positioning L234 at this site accommodated connectivity between the AQP0CBD and the transmembrane domain. A different hydrophobic residue within the AQP0CBD (such as L227 and/or V230) may also act as a primary anchor, but this would require a CaM conformation that is unique from the ptGAD–CaM complex. (Step 5) The resulting structure of CaM bound to two AQP0CBD α-helices was placed into each vacant lobe in the EM map, with the N-termini of the AQP0CBD α-helices facing the last transmembrane α-helix in AQP0. (Step 6) Linker domains (residues 223–226) were modeled connecting the transmembrane domain of AQP0 to each AQP0CBD α-helix (labeled, and shown in grey). The final model was subjected to energy minimization to remove steric interactions. A 25 Å map calculated from this model gave a crosscorrelation of 0.95 with the experimental map.
Supplementary Figure 3 Isothermal titration calorimetry (ITC) of CaM binding toAQP0CBD peptides.
(a) (top panels) Raw heats of binding obtained by ITC when CaM was mixed with AQP0CBD peptides (residue 223–242 from the cow sequence shown in Figure 2a) corresponding to the wildtype and alanine point mutations made at the conserved hydrophobic residues indicated. (bottom panels) Binding isotherms fitted to the raw data using two-state and single-state binding models as indicated. (b) Table of thermodynamic parameters obtained by fitting the ITC data to a two-state or single-state binding model (N = number of binding sites, Ka = association constant, ΔH = change in enthalpy, -TΔS = change in entropy, ΔG = Gibb's free energy, subscript 1 and 2 refer to the first and second binding step for data fit to a two-state model.
Supplementary Figure 4 Superposition of starting models for molecular dynamics simulations.
(a) Superposition showing the starting conformations of AQP0 for used for MD simulations. Two MD simulations were generated, one using AQP0 in complex with Calmodulin (AQP0Cam-bound; blue) and the second using AQP0 alone (AQP0CaM-free; yellow). For the CaMfree system, a starting conformation of the AQP0 tetramer (residues 5 to 239) in the absence of CaM was created by deleting the CaM coordinates from the AQP0–CaM complex. In this way, the starting conformations of AQP0 in the two simulations were identical (r.m.s.d. = 0.0 Å). (b) Zoom view, showing that for both systems, the CSII sites of AQP0 were unaltered from the original 2B6P model. Note that CaM is not shown in this overlay.
Supplementary Figure 5 Immunoblot analysis of AQP0 membrane expression in Xenopus oocytes.
Immunoblot analysis of AQP0 membrane expression in Xenopus oocytes.Full-length gel showing immunoblot analysis of AQP0 constructs corresponding to Figure 4e, (inset) within the main text. Each lane corresponds to the membrane fraction for cells of that were uninjected (UI) or injected with RNA for wildtype AQP0 (Y149) and AQP0 point mutants (G149), (L149) and (S149). Samples were separated on SDS-PAGE and blotted with AQP0 antibody (H-44).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 628 kb)
Dynamics of AQP0 CSII gating residue Tyr149.
The AQP0 protein is depicted as grey cartoon with the CSII residues (Tyr149 and Phe75) displayed as blue sticks and water molecules displayed as red and white atoms. Note the movement of Tyr149 (lower right) out of the pore that coincides with a rush of water molecules across the CSII gate. (MOV 4762 kb)
Rights and permissions
About this article
Cite this article
Reichow, S., Clemens, D., Freites, J. et al. Allosteric mechanism of water-channel gating by Ca2+–calmodulin. Nat Struct Mol Biol 20, 1085–1092 (2013). https://doi.org/10.1038/nsmb.2630
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2630
This article is cited by
-
Aquaporin water channels: roles beyond renal water handling
Nature Reviews Nephrology (2023)
-
Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 Å
Nature Communications (2020)
-
Proteomic analysis showing the signaling pathways involved in the rhizome enlargement process in Nelumbo nucifera
BMC Genomics (2019)
-
Understanding the mechanisms of soil water repellency from nanoscale to ecosystem scale: a review
Journal of Soils and Sediments (2019)
-
Biocompatibility of iron carbide and detection of metals ions signaling proteomic analysis via HPLC/ESI-Orbitrap
Nano Research (2017)