Abstract
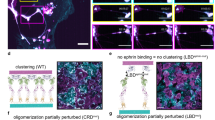
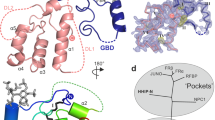
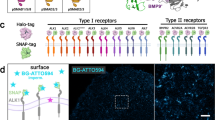
Functional outcomes of ephrin binding to Eph receptors (Ephs) range from cell repulsion to adhesion. Here we used cell collapse and stripe assays, showing contrasting effects of human ephrinA5 binding to EphA2 and EphA4. Despite equivalent ligand binding affinities, EphA4 triggered greater cell collapse, whereas EphA2-expressing cells adhered better to ephrinA5-coated surfaces. Chimeric receptors showed that the ectodomain is a major determinant of cell response. We report crystal structures of EphA4 ectodomain alone and in complexes with ephrinB3 and ephrinA5. These revealed closed clusters with a dimeric or circular arrangement in the crystal lattice, contrasting with extended arrays previously observed for EphA2 ectodomain. Localization microscopy showed that ligand-stimulated EphA4 induces smaller clusters than does EphA2. Mutant Ephs link these characteristics to interactions observed in the crystal lattices, suggesting a mechanism by which distinctive ectodomain surfaces determine clustering, and thereby signaling, properties.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Klein, R. Eph/ephrin signalling during development. Development 139, 4105–4109 (2012).
Batlle, E. & Wilkinson, D.G. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 4, 211–226 (2012).
Pitulescu, M.E. & Adams, R.H. Eph/ephrin molecules: a hub for signaling and endocytosis. Genes Dev. 24, 2480–2492 (2010).
Pasquale, E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10, 165–180 (2010).
Brantley–Sieders, D.M. Clinical relevance of Ephs and ephrins in cancer: lessons from breast, colorectal, and lung cancer profiling. Semin. Cell Dev. Biol. 23, 102–108 (2012).
Nievergall, E., Lackmann, M. & Janes, P.W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell Mol. Life Sci. 69, 1813–1842 (2012).
Gale, N.W. et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17, 9–19 (1996).
Bowden, T.A. et al. Structural plasticity of eph receptor A4 facilitates cross-class ephrin signaling. Structure 17, 1386–1397 (2009).
Noberini, R., Rubio de la Torre, E. & Pasquale, E.B. Profiling Eph receptor expression in cells and tissues: a targeted mass spectrometry approach. Cell Adh. Migr. 6, 102–112 (2012).
Himanen, J.P. Ectodomain structures of Eph receptors. Semin. Cell Dev. Biol. 23, 35–42 (2012).
Janes, P.W., Nievergall, E. & Lackmann, M. Concepts and consequences of Eph receptor clustering. Semin. Cell Dev. Biol. 23, 43–50 (2012).
Himanen, J.P. et al. Crystal structure of an Eph receptor–ephrin complex. Nature 414, 933–938 (2001).
Smith, F.M. et al. Dissecting the EphA3/Ephrin-A5 interactions using a novel functional mutagenesis screen. J. Biol. Chem. 279, 9522–9531 (2004).
Seiradake, E., Harlos, K., Sutton, G., Aricescu, A.R. & Jones, E.Y. An extracellular steric seeding mechanism for Eph–ephrin signaling platform assembly. Nat. Struct. Mol. Biol. 17, 398–402 (2010).
Himanen, J.P. et al. Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. USA 107, 10860–10865 (2010).
Egea, J. et al. Regulation of EphA 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function. Neuron 47, 515–528 (2005).
Dufour, A. et al. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron 39, 453–465 (2003).
Kullander, K. et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299, 1889–1892 (2003).
Kao, T.J., Law, C. & Kania, A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Semin. Cell Dev. Biol. 23, 83–91 (2012).
Wang, L., Klein, R., Zheng, B. & Marquardt, T. Anatomical coupling of sensory and motor nerve trajectory via axon tracking. Neuron 71, 263–277 (2011).
Brittis, P.A., Lu, Q. & Flanagan, J.G. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223–235 (2002).
Hafner, C. et al. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 50, 490–499 (2004).
Cooper, M.A. et al. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA 105, 16620–16625 (2008).
Qin, H. et al. Structural characterization of the EphA4–Ephrin-B2 complex reveals new features enabling Eph–ephrin binding promiscuity. J. Biol. Chem. 285, 644–654 (2010).
Poliakov, A., Cotrina, M. & Wilkinson, D.G. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell 7, 465–480 (2004).
Karplus, P.A. & Diederichs, K. Linking crystallographic model and data quality. Science 336, 1030–1033 (2012).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Lemmer, P. et al. SPDM: light microscopy with single-molecule resolution at the nanoscale. Appl. Phys. B 93, 1–12 (2008).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Edn Engl. 47, 6172–6176 (2008).
Ripley, B.D. Modeling spatial patterns. J. Roy. Stat. Soc. B Met 39, 172–212 (1977).
Kaufmann, R., Mueller, P., Hildenbrand, G., Hausmann, M. & Cremer, C. Analysis of Her2/neu membrane protein clusters in different types of breast cancer cells using localization microscopy. J. Microsc. 242, 46–54 (2010).
Wimmer-Kleikamp, S.H., Janes, P.W., Squire, A., Bastiaens, P.I. & Lackmann, M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 164, 661–666 (2004).
Triplett, J.W. & Feldheim, D.A. Eph and ephrin signaling in the formation of topographic maps. Semin. Cell Dev. Biol. 23, 7–15 (2012).
Astin, J.W. et al. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 12, 1194–1204 (2010).
Salaita, K. et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, 1380–1385 (2010).
Janes, P.W. et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J. Cell Biol. 195, 1033–1045 (2011).
Freywald, A., Sharfe, N. & Roifman, C.M. The kinase-null EphB6 receptor undergoes transphosphorylation in a complex with EphB1. J. Biol. Chem. 277, 3823–3828 (2002).
Miao, H. et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 16, 9–20 (2009).
Aricescu, A.R., Lu, W. & Jones, E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 (2006).
Reeves, P.J., Callewaert, N., Contreras, R. & Khorana, H.G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. USA 99, 13419–13424 (2002).
Grueninger-Leitch, F., D'Arcy, A., D'Arcy, B. & Chene, C. Deglycosylation of proteins for crystallization using recombinant fusion protein glycosidases. Protein Sci. 5, 2617–2622 (1996).
Walter, T.S. et al. Lysine methylation as a routine rescue strategy for protein crystallization. Structure 14, 1617–1622 (2006).
Walter, T.S. et al. A procedure for setting up high-throughput nanolitre crystallization experiments: crystallization workflow for initial screening, automated storage, imaging and optimization. Acta Crystallogr. D Biol. Crystallogr. 61, 651–657 (2005).
Winter, G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 43, 186–190 (2010).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Leslie, A.G. The integration of macromolecular diffraction data. Acta Crystallogr. D Biol. Crystallogr. 62, 48–57 (2006).
Collaborative Computational Project 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
DiMaio, F., Tyka, M.D., Baker, M.L., Chiu, W. & Baker, D. Refinement of protein structures into low-resolution density maps using rosetta. J. Mol. Biol. 392, 181–190 (2009).
Zwart, P.H. et al. Automated structure solution with the PHENIX suite. Methods Mol. Biol. 426, 419–435 (2008).
Zhang, K.Y., Cowtan, K. & Main, P. Combining constraints for electron-density modification. Methods Enzymol. 277, 53–64 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Blanc, E. et al. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D Biol. Crystallogr. 60, 2210–2221 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Xu, K. et al. Host cell recognition by the henipaviruses: crystal structures of the Nipah G attachment glycoprotein and its complex with ephrin-B3. Proc. Natl. Acad. Sci. USA 105, 9953–9958 (2008).
Adams, P.D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Gruell, F., Kirchgessner, M., Kaufmann, R., Hausmann, M. & Kebschull, U. Accelerating image analysis for localization microscopy with FPGAs. in International Conference on Field Programmable Logic and Applications 1–5 (Institute of Electrical and Electronics Engineers, 2011).
Knöll, B., Weinl, C., Nordheim, A. & Bonhoeffer, F. Stripe assay to examine axonal guidance and cell migration. Nat. Protoc. 2, 1216–1224 (2007).
Acknowledgements
We thank Y. Zhao and W. Lu for protein expression and M. Jones and T.S. Walter for technical support. We are grateful to T. Gaitanos for help with confocal microscopy data acquisition, I. Davis and I.M. Dobbie for discussion and assistance at the single-molecule–localization facility in the Micron Advanced Bioimaging Unit, J. Erl and M. Ponserre for assistance with cell-based assays, K.J. Morris for providing access to MetaMorph software and R.M. Esnouf for aiding in protein structure analysis. We thank the staff of the Diamond Light Source for assistance with diffraction data collection and K. Diederichs for help with data integration. This research was funded by a Cancer Research United Kingdom grant to E.Y.J. (grant A10979). Localization microscopy facilities in the Micron Advanced Bioimaging Unit were funded by the Wellcome Trust (grant 091911). E.S. was funded by an Intra-European Fellowship (Marie Curie); D.d.T.R. was funded by an European Molecular Biology Organization long-term fellowship; N.M. is supported by a Wellcome Trust D.Phil. studentship; and A.R.A. was supported as a United Kingdom Medical Research Council Career Development Award Fellow. Partial funding was provided by the Deutsche Forschungsgemeinschaft (SFB870) to R. Klein and Wellcome Trust grant 090532/Z/09/Z supporting the Wellcome Trust Centre for Human Genetics.
Author information
Authors and Affiliations
Contributions
E.S. performed protein crystallization, structure analysis, cell rounding assays and Eph clustering experiments. A.S. contributed to time-lapse imaging experiments. D.d.T.R. performed stripe assays. R. Kaufman conducted localization microscopy data acquisition and analysis. N.M. contributed to protein crystallization. K.H. performed Eph crystal mounting for data collection. A.R.A., R. Klein and E.Y.J. contributed to discussion at all stages of the project. All authors contributed to writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Note (PDF 3739 kb)
Supplementary Movie 1
Phase-contrast images showing the response of an EphA2-mVenus transfected cell upon pre-clustered ephrinA5-Fc stimulation. Images were taken before and every 6 minutes after stimulation. (MOV 108 kb)
Supplementary Movie 2
Phase-contrast images showing the response of an EphA4-mVenus transfected cell upon pre-clustered ephrinA5-Fc stimulation. Images were taken before and every 6 minutes after stimulation. (MOV 157 kb)
Supplementary Movie 3
Confocal images of live COS7 cell time lapse experiments show mVenus-tagged EphA2 clustering upon ephrinA5-Fc stimulation. (MOV 275 kb)
Supplementary Movie 4
Confocal images of live COS7 cell time lapse experiments show mVenus-tagged EphA4 clustering upon ephrinA5-Fc stimulation. (MOV 908 kb)
Supplementary Movie 5
Confocal images of live COS7 cell time lapse experiments show mVenus-tagged chimeric Eph receptor A4A2 clustering upon ephrinA5-Fc stimulation. A4A2 contains an EphA4 ectodomain fused to EphA2 transmembrane and intracellular domains. (MOV 247 kb)
Supplementary Movie 6
Confocal images of live COS7 cell time lapse experiments show mVenus-tagged chimeric Eph receptor A2A4 clustering upon ephrinA5-Fc stimulation. A2A4 contains an EphA2 ectodomain fused to EphA4 transmembrane and intracellular domains. (MOV 434 kb)
Supplementary Movie 7
Confocal images of live COS7 cell time lapse experiments show the mVenus-tagged EphA2 sushi dimerization surface mutant (EphA2su) clustering upon ephrinA5-Fc stimulation. (MOV 284 kb)
Supplementary Movie 8
Confocal images of live COS7 cell time lapse experiments show the mVenus-tagged EphA2 sushi dimerization surface mutant (EphA2su) clustering upon ephrinA5-Fc stimulation. (MOV 401 kb)
Supplementary Movie 9
mCherry-tagged EphA2 was co-expressed in COS7 cells with the non-ephrin-binding mVenus-tagged mutant EphA2nb. Confocal images of time lapse experiments show mVenus-tagged EphA2nb co-clustering with mCherry-tagged EphA2 upon ephrinA5-Fc stimulation. (MOV 330 kb)
Supplementary Movie 10
mCherry-tagged EphA2 was co-expressed in COS7 cells with the mVenus-tagged non-ephrin-binding and sushi dimerization surface mutant EphA2nb–su. Confocal images of time lapse experiments show mVenus-tagged EphA2nb–su does not co-cluster with mCherry-tagged EphA2 upon ephrinA5-Fc stimulation. (MOV 352 kb)
Rights and permissions
About this article
Cite this article
Seiradake, E., Schaupp, A., del Toro Ruiz, D. et al. Structurally encoded intraclass differences in EphA clusters drive distinct cell responses. Nat Struct Mol Biol 20, 958–964 (2013). https://doi.org/10.1038/nsmb.2617
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2617
This article is cited by
-
Eph receptors and ephrins in cancer progression
Nature Reviews Cancer (2024)
-
Targeting EphA2: a promising strategy to overcome chemoresistance and drug resistance in cancer
Journal of Molecular Medicine (2024)
-
Glycoprotein attachment with host cell surface receptor ephrin B2 and B3 in mediating entry of nipah and hendra virus: a computational investigation
Journal of Chemical Sciences (2022)
-
Regulation of the EphA2 receptor intracellular region by phosphomimetic negative charges in the kinase-SAM linker
Nature Communications (2021)
-
Single-cell analysis of EphA clustering phenotypes to probe cancer cell heterogeneity
Communications Biology (2020)