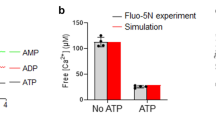
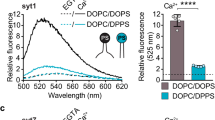
Abstract
Synaptic-vesicle exocytosis is mediated by the vesicular Ca2+ sensor synaptotagmin-1. Synaptotagmin-1 interacts with the SNARE protein syntaxin-1A and acidic phospholipids such as phosphatidylinositol 4,5-bisphosphate (PIP2). However, it is unclear how these interactions contribute to triggering membrane fusion. Using PC12 cells from Rattus norvegicus and artificial supported bilayers, we show that synaptotagmin-1 interacts with the polybasic linker region of syntaxin-1A independent of Ca2+ through PIP2. This interaction allows both Ca2+-binding sites of synaptotagmin-1 to bind to phosphatidylserine in the vesicle membrane upon Ca2+ triggering. We determined the crystal structure of the C2B domain of synaptotagmin-1 bound to phosphoserine, allowing development of a high-resolution model of synaptotagmin bridging two different membranes. Our results suggest that PIP2 clusters organized by syntaxin-1 act as molecular beacons for vesicle docking, with the subsequent Ca2+ influx bringing the vesicle membrane close enough for membrane fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Südhof, T.C. & Rothman, J.E. Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 (2009).
Rizo, J., Chen, X. & Araç, D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 16, 339–350 (2006).
Chapman, E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 (2008).
Jahn, R. & Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 490, 201–207 (2012).
Sieber, J.J. et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317, 1072–1076 (2007).
Aoyagi, K. et al. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 280, 17346–17352 (2005).
van den Bogaart, G. et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 (2011).
Choi, U.B. et al. Single-molecule FRET–derived model of the synaptotagmin 1–SNARE fusion complex. Nat. Struct. Mol. Biol. 17, 318–324 (2010).
Araç, D. et al. Facile detection of protein–protein interactions by one-dimensional NMR spectroscopy. Biochemistry 42, 2774–2780 (2003).
Lai, A.L., Huang, H., Herrick, D.Z., Epp, N. & Cafiso, D.S. Synaptotagmin 1 and SNAREs form a complex that is structurally heterogeneous. J. Mol. Biol. 405, 696–706 (2011).
Tang, J. et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 (2006).
Xue, M., Ma, C., Craig, T.K., Rosenmund, C. & Rizo, J. The Janus-faced nature of the C2B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15, 1160–1168 (2008).
Radhakrishnan, A., Stein, A., Jahn, R. & Fasshauer, D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284, 25749–25760 (2009).
McMahon, H.T., Kozlov, M.M. & Martens, S. Membrane curvature in synaptic vesicle fusion and beyond. Cell 140, 601–605 (2010).
Li, L. et al. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J. Biol. Chem. 281, 15845–15852 (2006).
Schiavo, G., Gu, Q.M., Prestwich, G.D., Söllner, T.H. & Rothman, J.E. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. USA 93, 13327–13332 (1996).
van den Bogaart, G. et al. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat. Struct. Mol. Biol. 18, 805–812 (2011).
van den Bogaart, G., Meyenberg, K., Diederichsen, U. & Jahn, R. Phosphatidylinositol 4,5-bisphosphate increases the Ca2+ affinity of synaptotagmin-1 40-fold. J. Biol. Chem. 287, 16447–16453 (2012).
Araç, D. et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Hui, E. et al. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat. Struct. Mol. Biol. 18, 813–821 (2011).
Herrick, D.Z., Sterbling, S., Rasch, K.A., Hinderliter, A. & Cafiso, D.S. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry 45, 9668–9674 (2006).
Connell, E. et al. Cross-linking of phospholipid membranes is a conserved property of calcium-sensitive synaptotagmins. J. Mol. Biol. 380, 42–50 (2008).
Kim, J.Y. et al. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 31, 2144–2155 (2012).
Kuo, W., Herrick, D.Z. & Cafiso, D.S. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry 50, 2633–2641 (2011).
Joung, M.J., Mohan, S.K. & Yu, C. Molecular level interaction of inositol hexaphosphate with the C2B domain of human synaptotagmin I. Biochemistry 51, 3675–3683 (2012).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Vrljic, M. et al. Post-translational modifications and lipid binding profile of insect cell-expressed full-length mammalian synaptotagmin 1. Biochemistry 50, 9998–10012 (2011).
Eggeling, C. et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162 (2009).
Mueller, V. et al. STED nanoscopy reveals molecular details of cholesterol- and cytoskeleton-modulated lipid interactions in living cells. Biophys. J. 101, 1651–1660 (2011).
Milosevic, I. et al. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25, 2557–2565 (2005).
Rickman, C. et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 (2004).
Jensen, M.H., Morris, E.J. & Simonsen, A.C. Domain shapes, coarsening, and random patterns in ternary membranes. Langmuir 23, 8135–8141 (2007).
Murray, D. et al. Electrostatic properties of membranes containing acidic lipids and adsorbed basic peptides: theory and experiment. Biophys. J. 77, 3176–3188 (1999).
Levental, I. et al. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry 48, 8241–8248 (2009).
Ausili, A., Corbalán-García, S., Gómez-Fernández, J.C. & Marsh, D. Membrane docking of the C2 domain from protein kinase Cα as seen by polarized ATR-IR. The role of PIP2 . Biochim. Biophys. Acta 1808, 684–695 (2011).
Lai, C.L., Landgraf, K.E., Voth, G.A. & Falke, J.J. Membrane docking geometry and target lipid stoichiometry of membrane-bound PKCα C2 domain: a combined molecular dynamics and experimental study. J. Mol. Biol. 402, 301–310 (2010).
Chen, C.H. et al. Configuration of PKCα-C2 domain bound to mixed SOPC/SOPS lipid monolayers. Biophys. J. 97, 2794–2802 (2009).
Montaville, P. et al. The PIP2 binding mode of the C2 domains of rabphilin-3A. Protein Sci. 17, 1025–1034 (2008).
Guerrero-Valero, M. et al. Structural and mechanistic insights into the association of PKCα-C2 domain to PtdIns(4,5)P2. Proc. Natl. Acad. Sci. USA 106, 6603–6607 (2009).
Verdaguer, N., Corbalan-Garcia, S., Ochoa, W.F., Fita, I. & Gómez-Fernández, J.C. Ca2+ bridges the C2 membrane-binding domain of protein kinase Cα directly to phosphatidylserine. EMBO J. 18, 6329–6338 (1999).
Cheng, Y. et al. Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin I C2B domain. Protein Sci. 13, 2665–2672 (2004).
Marrink, S.J., Risselada, H.J., Yefimov, S., Tieleman, D.P. & de Vries, A.H. The MARTINI forcefield: coarse grained model for biomolecular simulations. J. Phys. Chem. B 111, 7812–7824 (2007).
de Wit, H. et al. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell 138, 935–946 (2009).
Martin, T.F.J. Role of PI(4,5)P2 in vesicle exocytosis and membrane fusion. Subcell. Biochem. 59, 111–130 (2012).
Schneggenburger, R. & Neher, E. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 15, 266–274 (2005).
Kolmakov, K. et al. Red-emitting rhodamine dyes for fluorescence microscopy and nanoscopy. Chemistry. 16, 158–166 (2010).
Barnstable, C.J., Hofstein, R. & Akagawa, K. A marker of early amacrine cell development in rat retina. Brain Res. 352, 286–290 (1985).
Heumann, R., Kachel, V. & Thoenen, H. Relationship between NGF-mediated volume increase and “priming effect” in fast and slow reacting clones of PC12 pheochromocytoma cells. Role of cAMP. Exp. Cell Res. 145, 179–190 (1983).
van den Bogaart, G. et al. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 17, 358–364 (2010).
Mennicke, U. & Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 18, 8172–8177 (2002).
Vicidomini, G. et al. Sharper low-power STED nanoscopy by time gating. Nat. Methods 8, 571–573 (2011).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Long, F., Vagin, A.A., Young, P. & Murshudov, G.N. BALBES: a molecular-replacement pipeline. Acta Crystallogr. D Biol. Crystallogr. 64, 125–132 (2008).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P. & Cowtan, K. COOT: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Acknowledgements
We thank V. Belov (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for the KK114-maleimide dye and D. Cafiso (University of Virginia, Charlottesville, West Virginia, USA) for comments. G.v.d.B. is financed by the Human Frontier Science Program. This work was supported by the US National Institutes of Health (P01 GM072694, to R.J.) and the Deutsche Forschungsgemeinschaft (SFB803). We thank A. Schönle (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for the software ImSpector. X-ray diffraction data were collected at beamline X10SA at the Swiss Light Source, and we thank the beamline staff for their help during data collection.
Author information
Authors and Affiliations
Contributions
G.v.d.B., A.H., D.M. and R.J. designed and performed the experiments and wrote the paper. E.I., D.F. and K.K. performed the crystallographic structure determination. H.J.R. and H.G. performed the molecular dynamics simulations. S.M. and U.D. synthesized the peptide. S.W.H., V.M. and C.E. contributed to the microscopy and discussed data. All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Note (PDF 1457 kb)
Rights and permissions
About this article
Cite this article
Honigmann, A., van den Bogaart, G., Iraheta, E. et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat Struct Mol Biol 20, 679–686 (2013). https://doi.org/10.1038/nsmb.2570
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2570
This article is cited by
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Analysis of the MCTP Amino Acid Sequence Reveals the Conservation of Putative Calcium- and Lipid-Binding Pockets Within the C2 Domains In Silico
Journal of Molecular Evolution (2022)
-
Lysine acetylation regulates the interaction between proteins and membranes
Nature Communications (2021)
-
Insulin granule biogenesis and exocytosis
Cellular and Molecular Life Sciences (2021)
-
Cooperativity of membrane-protein and protein–protein interactions control membrane remodeling by epsin 1 and affects clathrin-mediated endocytosis
Cellular and Molecular Life Sciences (2021)