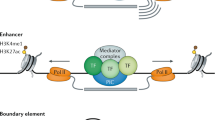
Abstract
Although genomes are defined by their sequence, the linear arrangement of nucleotides is only their most basic feature. A fundamental property of genomes is their topological organization in three-dimensional space in the intact cell nucleus. The application of imaging methods and genome-wide biochemical approaches, combined with functional data, is revealing the precise nature of genome topology and its regulatory functions in gene expression and genome maintenance. The emerging picture is one of extensive self-enforcing feedback between activity and spatial organization of the genome, suggestive of a self-organizing and self-perpetuating system that uses epigenetic dynamics to regulate genome function in response to regulatory cues and to propagate cell-fate memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Marina Corral


Marina Corral
Similar content being viewed by others
References
Lanctot, C., Cheutin, T., Cremer, M., Cavalli, G. & Cremer, T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat. Rev. Genet. 8, 104–115 (2007).
Misteli, T. Beyond the sequence: Cellular organization of genome function. Cell 128, 787–800 (2007).
Rajapakse, I. & Groudine, M. On emerging nuclear order. J. Cell Biol. 192, 711–721 (2011). This comprehensive overview of basic principles of genome organization includes a discussion of the concept of self-organized genome architecture.
Hakim, O. & Misteli, T. SnapShot: chromosome confirmation capture. Cell 148, 1068 e1–e2 (2012).
van Steensel, B. & Dekker, J. Genomics tools for unraveling chromosome architecture. Nat. Biotechnol. 28, 1089–1095 (2010).
Branco, M.R. & Pombo, A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 4, e138 (2006).
Zimmer, C. & Fabre, E. Principles of chromosomal organization: lessons from yeast. J. Cell Biol. 192, 723–733 (2011).
Sanyal, A., Lajoie, B.R., Jain, G. & Dekker, J. The long-range interaction landscape of gene promoters. Nature 489, 109–113 (2012). This work comprehensively maps interactions between transcription start sites and distal elements in 1% of the human genome to generate first insights into the spatial arrangements of genes and regulatory elements in their 3D context.
Chubb, J.R., Boyle, S., Perry, P. & Bickmore, W.A. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr. Biol. 12, 439–445 (2002).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Filion, G.J. et al. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 143, 212–224 (2010). This work characterizes five types of chromatin based on combinatorial enrichment of histone modifications and chromatin-binding proteins.
Kharchenko, P.V. et al. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471, 480–485 (2011). Refs. 12 and 15 are two of several reports published from the ModENCODE and the ENCODE projects, respectively. Similar to ref. 11, they highlight the existence of chromosomal domains characterized by distinct epigenomic landscapes in D. melanogaster and humans.
Liu, T. et al. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 21, 227–236 (2011).
Schwartz, Y.B. et al. Alternative epigenetic chromatin states of polycomb target genes. PLoS Genet. 6, e1000805 (2010).
Dunham, I. et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Caron, H. et al. The human transcriptome map: clustering of highly expressed genes in chromosomal domains. Science 291, 1289–1292 (2001).
Gierman, H.J. et al. Domain-wide regulation of gene expression in the human genome. Genome Res. 17, 1286–1295 (2007).
Dixon, J.R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012). Results reported in references 18–21 demonstrate the existence of topological domains in the D. melanogaster , mouse and human genomes. This work characterized the chromatin features at boundaries subdividing these domains as well as the nature of the long-distance contacts between different domains.
Hou, C., Li, L., Qin, Z.S. & Corces, V.G. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol. Cell 48, 471–484 (2012).
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012).
Nora, E.P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Shopland, L.S. et al. Folding and organization of a contiguous chromosome region according to the gene distribution pattern in primary genomic sequence. J. Cell Biol. 174, 27–38 (2006).
Boutanaev, A.M., Mikhaylova, L.M. & Nurminsky, D.I. The pattern of chromosome folding in interphase is outlined by the linear gene density profile. Mol. Cell. Biol. 25, 8379–8386 (2005).
Simonis, M. et al. High-resolution identification of balanced and complex chromosomal rearrangements by 4C technology. Nat. Methods 6, 837–842 (2009).
Noordermeer, D. et al. The dynamic architecture of Hox gene clusters. Science 334, 222–225 (2011).
Wong, H. et al. A predictive computational model of the dynamic 3D interphase yeast nucleus. Curr. Biol. 22, 1881–1890 (2012). Work reported in refs. 26 and 27 suggests that yeast higher-order nuclear architecture is largely driven by chromosome polymer structure.
Tjong, H., Gong, K., Chen, L. & Alber, F. Physical tethering and volume exclusion determine higher-order genome organization in budding yeast. Genome Res. 22, 1295–1305 (2012).
Guelen, L. et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008).
Kind, J. & van Steensel, B. Genome-nuclear lamina interactions and gene regulation. Curr. Opin. Cell Biol. 22, 320–325 (2010).
Wang, J. et al. Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J. Cell Biol. 164, 515–526 (2004).
Shopland, L.S. et al. Replication-dependent histone gene expression is related to Cajal body (CB) association but does not require sustained CB contact. Mol. Biol. Cell 12, 565–576 (2001).
Bantignies, F. et al. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell 144, 214–226 (2011).
Cheutin, T. & Cavalli, G. Progressive polycomb assembly on H3K27me3 compartments generates polycomb bodies with developmentally regulated motion. PLoS Genet. 8, e1002465 (2012).
Eskiw, C.H. et al. Transcription factories and nuclear organization of the genome. Cold Spring Harb. Symp. Quant. Biol. 75, 501–506 (2010).
Meaburn, K.J., Gudla, P.R., Khan, S., Lockett, S.J. & Misteli, T. Disease-specific gene repositioning in breast cancer. J. Cell Biol. 187, 801–812 (2009).
Kalhor, R., Tjong, H., Jayahilaka, N., Alber, F. & Chen, L. Solid-phase chromosome conformation capture for structural characterization of genome architectures. Nat. Biotechnol. 30, 90–98 (2012).
Cvackova, Z., Masata, M., Stanek, D., Fidlerova, H. & Raska, I. Chromatin position in human HepG2 cells: although being non-random, significantly changed in daughter cells. J. Struct. Biol. 165, 107–117 (2009).
Hou, C. & Corces, V.G. Throwing transcription for a loop: expression of the genome in the 3D nucleus. Chromosoma 121, 107–116 (2012).
O'Sullivan, J.M. et al. Gene loops juxtapose promoters and terminators in yeast. Nat. Genet. 36, 1014–1018 (2004).
Tan-Wong, S.M., French, J.D., Proudfoot, N.J. & Brown, M.A. Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proc. Natl. Acad. Sci. USA 105, 5160–5165 (2008).
Tan-Wong, S.M., Wijayatilake, H.D. & Proudfoot, N.J. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 23, 2610–2624 (2009).
Tan-Wong, S.M. et al. Gene loops enhance transcriptional directionality. Science 338, 671–675 (2012).
Tolhuis, B., Palstra, R.J., Splinter, E., Grosveld, F. & de Laat, W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell 10, 1453–1465 (2002).
Orom, U.A. & Shiekhattar, R. Noncoding RNAs and enhancers: complications of a long-distance relationship. Trends Genet. 27, 433–439 (2011).
Wang, K.C. et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011).
Deng, W. et al. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149, 1233–1244 (2012). This work demonstrates that chromatin looping causally underlies gene regulation, as determined by experimentally manipulating the formation of chromatin loops.
Vernimmen, D. et al. Polycomb eviction as a new distant enhancer function. Genes Dev. 25, 1583–1588 (2011).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Tiwari, V.K., Cope, L., McGarvey, K.M., Ohm, J.E. & Baylin, S.B. A novel 6C assay uncovers Polycomb-mediated higher order chromatin conformations. Genome Res. 18, 1171–1179 (2008).
Tiwari, V.K. et al. PcG proteins, DNA methylation, and gene repression by chromatin looping. PLoS Biol. 6, 2911–2927 (2008).
Lanzuolo, C., Roure, V., Dekker, J., Bantignies, F. & Orlando, V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat. Cell Biol. 9, 1167–1174 (2007).
Breiling, A., Turner, B.M., Bianchi, M.E. & Orlando, V. General transcription factors bind promoters repressed by Polycomb group proteins. Nature 412, 651–655 (2001).
Lanzuolo, C. & Orlando, V. The function of the epigenome in cell reprogramming. Cell. Mol. Life Sci. 64, 1043–1062 (2007).
Saurin, A.J., Shao, Z., Erdjument-Bromage, H., Tempst, P. & Kingston, R.E. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412, 655–660 (2001).
Cleard, F., Moshkin, Y., Karch, F. & Maeda, R.K. Probing long-distance regulatory interactions in the Drosophila melanogaster bithorax complex using Dam identification. Nat. Genet. 38, 931–935 (2006).
Li, B., Carey, M. & Workman, J.L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Wicks, K. & Knight, J.C. Transcriptional repression and DNA looping associated with a novel regulatory element in the final exon of the lymphotoxin-beta gene. Genes Immun. 12, 126–135 (2011).
Hsu, P.Y. et al. Estrogen-mediated epigenetic repression of large chromosomal regions through DNA looping. Genome Res. 20, 733–744 (2010).
Hewetson, A. & Chilton, B.S. Progesterone-dependent deoxyribonucleic acid looping between RUSH/SMARCA3 and Egr-1 mediates repression by c-Rel. Mol. Endocrinol. 22, 813–822 (2008).
Yang, J. & Corces, V.G. Insulators, long-range interactions, and genome function. Curr. Opin. Genet. Dev. 22, 86–92 (2012).
Thurman, R.E. et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82 (2012).
Van Bortle, K. et al. Drosophila CTCF tandemly aligns with other insulator proteins at the borders of H3K27me3 domains. Genome Res. 22, 2176–2187 (2012).
Schwartz, Y.B. et al. Nature and function of insulator protein binding sites in the Drosophila genome. Genome Res. 22, 2188–2198 (2012).
Masui, O. et al. Live-cell chromosome dynamics and outcome of X chromosome pairing events during ES cell differentiation. Cell 145, 447–458 (2011).
Augui, S. et al. Sensing X chromosome pairs before X inactivation via a novel X-pairing region of the Xic. Science 318, 1632–1636 (2007).
Zhang, Y. et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148, 908–921 (2012).
Zhao, Z. et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nat. Genet. 38, 1341–1347 (2006).
Sandhu, K.S. et al. Nonallelic transvection of multiple imprinted loci is organized by the H19 imprinting control region during germline development. Genes Dev. 23, 2598–2603 (2009).
Ling, J.Q. et al. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312, 269–272 (2006).
Schoenfelder, S. et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat. Genet. 42, 53–61 (2010).
Dundr, M. & Misteli, T. Biogenesis of nuclear bodies. Cold Spring Harb. Perspect. Biol. 2, a000711 (2010).
Noordermeer, D. et al. Variegated gene expression caused by cell-specific long-range DNA interactions. Nat. Cell Biol. 13, 944–951 (2011).
Spilianakis, C.G., Lalioti, M.D., Town, T., Lee, G.R. & Flavell, R.A. Interchromosomal associations between alternatively expressed loci. Nature 435, 637–645 (2005).
Lomvardas, S. et al. Interchromosomal interactions and olfactory receptor choice. Cell 126, 403–413 (2006).
Clowney, E.J. et al. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell 151, 724–737 (2012). This work reveals that, in mouse olfactory neurons, silent olfactory receptor genes from different chromosomes converge in a small number of heterochromatic foci, suggesting that monogenic and monoallelic expression of olfactory receptors may be dependent on nuclear organization.
Apostolou, E. & Thanos, D. Virus infection induces NF-κB-dependent interchromosomal associations mediating monoallelic IFN-β gene expression. Cell 134, 85–96 (2008).
Kumaran, R.I. & Spector, D.L. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J. Cell Biol. 180, 51–65 (2008).
Finlan, L.E. et al. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 4, e1000039 (2008).
Reddy, K.L., Zullo, J.M., Bertolino, E. & Singh, H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452, 243–247 (2008).
Zullo, J.M. et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 149, 1474–1487 (2012).
Egecioglu, D. & Brickner, J.H. Gene positioning and expression. Curr. Opin. Cell Biol. 23, 338–345 (2011).
Towbin, B.D. et al. Step-wise methylation of Histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947 (2012).
Peric-Hupkes, D. et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 38, 603–613 (2010).
Kubben, N. et al. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma 121, 447–464 (2012).
Yokochi, T. et al. G9a selectively represses a class of late-replicating genes at the nuclear periphery. Proc. Natl. Acad. Sci. USA 106, 19363–19368 (2009).
Mechali, M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat. Rev. Mol. Cell Biol. 11, 728–738 (2010).
Schwaiger, M. et al. Chromatin state marks cell-type- and gender-specific replication of the Drosophila genome. Genes Dev. 23, 589–601 (2009).
Cadoret, J.C. et al. Genome-wide studies highlight indirect links between human replication origins and gene regulation. Proc. Natl. Acad. Sci. USA 105, 15837–15842 (2008).
Cayrou, C., Gregoire, D., Coulombe, P., Danis, E. & Mechali, M. Genome-scale identification of active DNA replication origins. Methods 57, 158–164 (2012).
Danis, E. et al. Specification of a DNA replication origin by a transcription complex. Nat. Cell Biol. 6, 721–730 (2004).
Schubeler, D. et al. Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nat. Genet. 32, 438–442 (2002).
Hiratani, I., Takebayashi, S., Lu, J. & Gilbert, D.M. Replication timing and transcriptional control: beyond cause and effect–part II. Curr. Opin. Genet. Dev. 19, 142–149 (2009).
Gilbert, D.M. Replication timing and transcriptional control: beyond cause and effect. Curr. Opin. Cell Biol. 14, 377–383 (2002).
Hiratani, I. et al. Global reorganization of replication domains during embryonic stem cell differentiation. PLoS Biol. 6, e245 (2008). Work described in refs. 94 and 95 reveals a tight correlation between the timing of replication in large chromosomal domains and their spatial organization as assessed by 3C technologies.
Ryba, T. et al. Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res. 20, 761–770 (2010).
Moindrot, B. et al. 3D chromatin conformation correlates with replication timing and is conserved in resting cells. Nucleic Acids Res. 40, 9470–9481 (2012).
Takebayashi, S., Dileep, V., Ryba, T., Dennis, J.H. & Gilbert, D.M. Chromatin-interaction compartment switch at developmentally regulated chromosomal domains reveals an unusual principle of chromatin folding. Proc. Natl. Acad. Sci. USA 109, 12574–12579 (2012).
Gilbert, D.M. Cell fate transitions and the replication timing decision point. J. Cell Biol. 191, 899–903 (2010).
Goodarzi, A.A. et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell 31, 167–177 (2008).
Cowell, I.G. et al. gammaH2AX foci form preferentially in euchromatin after ionising-radiation. PLoS ONE 2, e1057 (2007).
Kruhlak, M.J. et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J. Cell Biol. 172, 823–834 (2006).
Ayoub, N., Jeyasekharan, A.D., Bernal, J.A. & Venkitaraman, A.R. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 453, 682–686 (2008).
Ziv, Y. et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat. Cell Biol. 8, 870–876 (2006).
Chiolo, I. et al. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144, 732–744 (2011).
Soutoglou, E. et al. Positional stability of single double-strand breaks in mammalian cells. Nat. Cell Biol. 9, 675–682 (2007).
Roix, J.J., McQueen, P.G., Munson, P.J., Parada, L.A. & Misteli, T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat. Genet. 34, 287–291 (2003). This work provides extensive evidence for a key role of spatial genome organization in determining chromosome translocations.
Hakim, O. et al. DNA damage defines sites of recurrent chromosomal translocations in B lymphocytes. Nature 484, 69–74 (2012).
Klein, I.A. et al. Translocation-capture sequencing reveals the extent and nature of chromosomal rearrangements in B lymphocytes. Cell 147, 95–106 (2011).
Chiarle, R. et al. Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell 147, 107–119 (2011).
Dion, V., Kalck, V., Horigome, C., Towbin, B.D. & Gasser, S.M. Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat. Cell Biol. 14, 502–509 (2012).
Mine-Hattab, J. & Rothstein, R. Increased chromosome mobility facilitates homology search during recombination. Nat. Cell Biol. 14, 510–517 (2012).
Mekhail, K., Seebacher, J., Gygi, S.P. & Moazed, D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 456, 667–670 (2008).
Bressan, D.A., Vazquez, J. & Haber, J.E. Mating type-dependent constraints on the mobility of the left arm of yeast chromosome III. J. Cell Biol. 164, 361–371 (2004).
Parada, L., McQueen, P. & Misteli, T. Tissue-specific spatial organization of genomes. Genome Biol. 5, R44 (2004).
Mathas, S. et al. Gene deregulation and spatial genome reorganization near breakpoints prior to formation of translocations in anaplastic large cell lymphoma. Proc. Natl. Acad. Sci. USA 106, 5831–5836 (2009).
Lin, C. et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell 139, 1069–1083 (2009).
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell 10, 105–116 (2006).
Mikkelsen, T.S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Efroni, S. et al. Global transcription in pluripotent embryonic stem cells. Cell Stem Cell 2, 437–447 (2008).
Fussner, E. et al. Constitutive heterochromatin reorganization during somatic cell reprogramming. EMBO J. 30, 1778–1789 (2011).
Gaspar-Maia, A. et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature 460, 863–868 (2009).
Ho, L. et al. esBAF facilitates pluripotency by conditioning the genome for LIF/STAT3 signalling and by regulating polycomb function. Nat. Cell Biol. 13, 903–913 (2011).
Biran, A. & Meshorer, E. Concise review: chromatin and genome organization in reprogramming. Stem Cells 30, 1793–1799 (2012).
Puschendorf, M. et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 40, 411–420 (2008).
Meister, P., Towbin, B.D., Pike, B.L., Ponti, A. & Gasser, S.M. The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev. 24, 766–782 (2010). This study reveals a developmental stage–specific and cell differentiation–specific regulation of gene positioning in the 3D space of the nucleus in C. elegans , opening the way to genetic dissection of developmental determinants of nuclear organization and function.
Ishimi, Y. et al. Changes in chromatin structure during aging of human skin fibroblasts. Exp. Cell Res. 169, 458–467 (1987).
O'Sullivan, R.J., Kubicek, S., Schreiber, S.L. & Karlseder, J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 17, 1218–1225 (2010).
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006).
O'Sullivan, R.J. & Karlseder, J. The great unravelling: chromatin as a modulator of the aging process. Trends Biochem. Sci. 37, 466–476 (2012).
Pegoraro, G. et al. Aging-related chromatin defects via loss of the NURD complex. Nat. Cell Biol. 11, 1261–1267 (2009).
Feser, J. et al. Elevated histone expression promotes life span extension. Mol. Cell 39, 724–735 (2010).
Sutton, A., Bucaria, J., Osley, M.A. & Sternglanz, R. Yeast ASF1 protein is required for cell cycle regulation of histone gene transcription. Genetics 158, 587–596 (2001).
Lesur, I. & Campbell, J.L. The transcriptome of prematurely aging yeast cells is similar to that of telomerase-deficient cells. Mol. Biol. Cell 15, 1297–1312 (2004).
Burgess, R.C., Misteli, T. & Oberdoerffer, P. DNA damage, chromatin, and transcription: the trinity of aging. Curr. Opin. Cell Biol. 24, 724–730 (2012).
Oberdoerffer, P. et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918 (2008).
Acknowledgements
G.C. was supported by the European Research Council (ERC-2008-AdG No 232947), the Centre National de la Recherche Scientifique, the European Network of Excellence EpiGeneSys and the Agence Nationale de la Recherche. T.M. was supported by the Intramural Research Program of the US National Institutes of Health, the National Cancer Institute, the Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cavalli, G., Misteli, T. Functional implications of genome topology. Nat Struct Mol Biol 20, 290–299 (2013). https://doi.org/10.1038/nsmb.2474
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2474
This article is cited by
-
Epigenetic analyses in forensic medicine: future and challenges
International Journal of Legal Medicine (2024)
-
Determining chromatin architecture with Micro Capture-C
Nature Protocols (2023)
-
A comparison of topologically associating domain callers over mammals at high resolution
BMC Bioinformatics (2022)
-
Homologous chromosome associations in domains before meiosis could facilitate chromosome recognition and pairing in wheat
Scientific Reports (2022)
-
TADfit is a multivariate linear regression model for profiling hierarchical chromatin domains on replicate Hi-C data
Communications Biology (2022)