Abstract
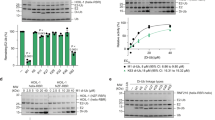
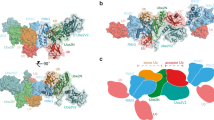
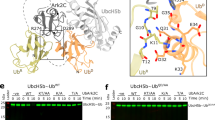
Mammalian RNF4 is a dimeric RING ubiquitin E3 ligase that ubiquitylates poly-SUMOylated proteins. We found that RNF4 bound ubiquitin-charged UbcH5a tightly but free UbcH5a weakly. To provide insight into the mechanism of RING-mediated ubiquitylation, we docked the UbcH5~ubiquitin thioester onto the RNF4 RING structure. This revealed that with E2 bound to one monomer of RNF4, the thioester-linked ubiquitin could reach across the dimer to engage the other monomer. In this model, the 'Ile44 hydrophobic patch' of ubiquitin is predicted to engage a conserved tyrosine located at the dimer interface of the RING, and mutation of these residues blocked ubiquitylation activity. Thus, dimeric RING ligases are not simply inert scaffolds that bring substrate and E2-loaded ubiquitin into close proximity. Instead, they facilitate ubiquitin transfer by preferentially binding the E2~ubiquitin thioester across the dimer and activating the thioester bond for catalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Dye, B.T. & Schulman, B.A. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu. Rev. Biophys. Biomol. Struct. 36, 131–150 (2007).
Pickart, C.M. & Eddins, M.J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 (2004).
Deshaies, R.J. & Joazeiro, C.A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 (2009).
Hay, R.T. SUMO: a history of modification. Mol. Cell 18, 1–12 (2005).
Perry, J.J., Tainer, J.A. & Boddy, M.N. A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33, 201–208 (2008).
Kosoy, A., Calonge, T.M., Outwin, E.A. & O'Connell, M.J. Fission yeast Rnf4 homologs are required for DNA repair. J. Biol. Chem. 282, 20388–20394 (2007).
Prudden, J. et al. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 26, 4089–4101 (2007).
Sun, H., Leverson, J.D. & Hunter, T. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 26, 4102–4112 (2007).
Uzunova, K. et al. Ubiquitin-dependent proteolytic control of SUMO conjugates. J. Biol. Chem. 282, 34167–34175 (2007).
Xie, Y. et al. The yeast Hex3.Slx8 heterodimer is a ubiquitin ligase stimulated by substrate sumoylation. J. Biol. Chem. 282, 34176–34184 (2007).
Häkli, M., Lorick, K.L., Weissman, A.M., Janne, O.A. & Palvimo, J.J. Transcriptional coregulator SNURF (RNF4) possesses ubiquitin E3 ligase activity. FEBS Lett. 560, 56–62 (2004).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008).
Tatham, M.H. et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 10, 538–546 (2008).
Song, J., Durrin, L.K., Wilkinson, T.A., Krontiris, T.G. & Chen, Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. USA 101, 14373–14378 (2004).
Hecker, C.M., Rabiller, M., Haglund, K., Bayer, P. & Dikic, I. Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281, 16117–16127 (2006).
Song, J., Zhang, Z., Hu, W. & Chen, Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J. Biol. Chem. 280, 40122–40129 (2005).
Liew, C.W., Sun, H., Hunter, T. & Day, C.L. RING domain dimerization is essential for RNF4 function. Biochem. J. 431, 23–29 (2010).
Mace, P.D. et al. Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 283, 31633–31640 (2008).
Linke, K. et al. Structure of the MDM2/MDMX RING domain heterodimer reveals dimerization is required for their ubiquitylation in trans. Cell Death Differ. 15, 841–848 (2008).
Vander Kooi, C.W. et al. The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 45, 121–130 (2006).
Yin, Q. et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 16, 658–666 (2009).
Wu, P.Y. et al. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 22, 5241–5250 (2003).
Sakata, E. et al. Crystal structure of UbcH5b~ubiquitin intermediate: insight into the formation of the self-assembled E2~Ub conjugates. Structure 18, 138–147 (2010).
Dikic, I., Wakatsuki, S. & Walters, K.J. Ubiquitin-binding domains—from structures to functions. Nat. Rev. Mol. Cell Biol. 10, 659–671 (2009).
Lee, S. et al. Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5. Nat. Struct. Mol. Biol. 13, 264–271 (2006).
Penengo, L. et al. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell 124, 1183–1195 (2006).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Mastrandrea, L.D., Kasperek, E.M., Niles, E.G. & Pickart, C.M. Core domain mutation (S86Y) selectively inactivates polyubiquitin chain synthesis catalyzed by E2–25K. Biochemistry 37, 9784–9792 (1998).
Brzovic, P.S., Lissounov, A., Christensen, D.E., Hoyt, D.W. & Klevit, R.E.A. UbcH5/ubiquitin noncovalent complex is required for processive BRCA1-directed ubiquitination. Mol. Cell 21, 873–880 (2006).
Christensen, D.E., Brzovic, P.S. & Klevit, R.E. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat. Struct. Mol. Biol. 14, 941–948 (2007).
Kleiger, G., Saha, A., Lewis, S., Kuhlman, B. & Deshaies, R.J. Rapid E2–E3 assembly and disassembly enable processive ubiquitylation of cullin-RING ubiquitin ligase substrates. Cell 139, 957–968 (2009).
Pierce, N.W., Kleiger, G., Shan, S.O. & Deshaies, R.J. Detection of sequential polyubiquitylation on a millisecond timescale. Nature 462, 615–619 (2009).
Brzovic, P.S., Rajagopal, P., Hoyt, D.W., King, M.C. & Klevit, R.E. Structure of a BRCA1-BARD1 heterodimeric RING-RING complex. Nat. Struct. Biol. 8, 833–837 (2001).
Buchwald, G. et al. Structure and E3-ligase activity of the Ring-Ring complex of Polycomb proteins Bmi1 and Ring1b. EMBO J. 25, 2465–2474 (2006).
Xu, Z. et al. Interactions between the quality control ubiquitin ligase CHIP and ubiquitin conjugating enzymes. BMC Struct. Biol. 8, 26 (2008).
Zhang, M. et al. Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell 20, 525–538 (2005).
Kamadurai, H.B. et al. Insights into ubiquitin transfer cascades from a structure of a UbcH5B~ubiquitin-HECT(NEDD4L) complex. Mol. Cell 36, 1095–1102 (2009).
Levin, I. et al. Identification of an unconventional E3 binding surface on the UbcH5~Ub conjugate recognized by a pathogenic bacterial E3 ligase. Proc. Natl. Acad. Sci. USA 107, 2848–2853 (2010).
Pruneda, J.N., Stoll, K.E., Bolton, L.J., Brzovic, P.S. & Klevit, R.E. Ubiquitin in motion: structural studies of the ubiquitin-conjugating enzyme~ubiquitin conjugate. Biochemistry 50, 1624–1633 (2011).
Wickliffe, K.E., Lorenz, S., Wemmer, D.E., Kuriyan, J. & Rape, M. The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell 144, 769–781 (2011).
Saha, A., Lewis, S., Kleiger, G., Kuhlman, B. & Deshaies, R.J. Essential role for ubiquitin-ubiquitin-conjugating enzyme interaction in ubiquitin discharge from Cdc34 to substrate. Mol. Cell 42, 75–83 (2011).
Martin, S.F., Tatham, M.H., Hay, R.T. & Samuel, I.D. Quantitative analysis of multi-protein interactions using FRET: application to the SUMO pathway. Protein Sci. 17, 777–784 (2008).
Martin, S.F., Hattersley, N., Samuel, I.D., Hay, R.T. & Tatham, M.H. A fluorescence-resonance-energy-transfer-based protease activity assay and its use to monitor paralog-specific small ubiquitin-like modifier processing. Anal. Biochem. 363, 83–90 (2007).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Morris, R.J., Perrakis, A. & Lamzin, V.S. ARP/wARP and automatic interpretation of protein electron density maps. Methods Enzymol. 374, 229–244 (2003).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 (2001).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–83 (2007).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Acknowledgements
We thank C. Botting for mass spectrometric analysis, M. Agacan for analytical ultracentrifugation analysis and N. Wood for help with cloning. His-Ube1 was a kind gift from the Division of Signal Transduction Therapy, University of Dundee. The sequence encoding RNF4 ΔC in pLou3 vector was a kind gift from L. Shen, University of Dundee. A.P. was funded by a studentship from the Wellcome Trust. This work was supported by a grant to R.T.H. from Cancer Research UK. The structural biology was supported by the Scottish Funding Council (reference SULSA) and by the UK Biotechnology and Biological Sciences Research Council through the SPoRT initiative.
Author information
Authors and Affiliations
Contributions
A.P. purified RNF4 proteins, carried out crystallography, conducted biochemical analysis and interpreted the data. E.G.J. purified recombinant proteins and carried out ubiquitylation assays. S.A.M., K.A.J. and J.H.N. contributed to structural analysis. I.N. contributed to biochemical analysis. A.P., J.H.N. and R.T.H. wrote the paper. R.T.H. conceived the project and contributed to data interpretation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Methods (PDF 4007 kb)
Rights and permissions
About this article
Cite this article
Plechanovová, A., Jaffray, E., McMahon, S. et al. Mechanism of ubiquitylation by dimeric RING ligase RNF4. Nat Struct Mol Biol 18, 1052–1059 (2011). https://doi.org/10.1038/nsmb.2108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2108
This article is cited by
-
UBE2A and UBE2B are recruited by an atypical E3 ligase module in UBR4
Nature Structural & Molecular Biology (2024)
-
Ubiquitin ligases: guardians of mammalian development
Nature Reviews Molecular Cell Biology (2022)
-
Production and characterisation of modularly deuterated UBE2D1–Ub conjugate by small angle neutron and X-ray scattering
European Biophysics Journal (2022)
-
Linkage-specific ubiquitin chain formation depends on a lysine hydrocarbon ruler
Nature Chemical Biology (2021)
-
Critical role of cysteine-266 of SIE3 in regulating the ubiquitination and degradation of SIP1 transcription factor in Lotus japonicus
Planta (2021)