Abstract
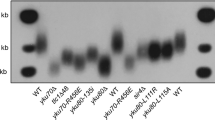
Telomere length homeostasis is an important aspect of telomere biology. Here, we show that SUMOylation limits telomere length and targets multiple telomere proteins in Saccharomyces cerevisiae. A main target is Cdc13, which both positively and negatively regulates telomerase and confers end protection. We demonstrate that Cdc13 SUMOylation restrains telomerase functions by promoting Cdc13 interaction with the telomerase inhibitor Stn1 without affecting end protection. Mutation of the Cdc13 SUMOylation site (cdc13-snm) lengthens telomeres and reduces the Stn1 interaction, whereas Cdc13-SUMO fusion has the opposite effects. cdc13-snm's effect on telomere length is epistatic with stn1, but not with yku70, tel1 or est1 alleles, and is suppressed by Stn1 overexpression. Cdc13 SUMOylation peaks in early-mid S phase, prior to its known Cdk1-mediated phosphorylation, and the two modifications act antagonistically, suggesting that the opposite roles of Cdc13 in telomerase regulation can be separated temporally and regulated by distinct modifications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bianchi, A. & Shore, D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol. Cell 31, 153–165 (2008).
Giraud-Panis, M.J., Teixeira, M.T., Geli, V. & Gilson, E. CST meets shelterin to keep telomeres in check. Mol. Cell 39, 665–676 (2010).
Wellinger, R.J., Wolf, A.J. & Zakian, V.A. Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell 72, 51–60 (1993).
Marcand, S., Brevet, V., Mann, C. & Gilson, E. Cell cycle restriction of telomere elongation. Curr. Biol. 10, 487–490 (2000).
Frank, C.J., Hyde, M. & Greider, C.W. Regulation of telomere elongation by the cyclin-dependent kinase Cdk1. Mol. Cell 24, 423–432 (2006).
Li, S. et al. Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell 136, 50–61 (2009).
Tseng, S.F., Shen, Z.J., Tsai, H.J., Lin, Y.H. & Teng, S.C. Rapid Cdc13 turnover and telomere length homeostasis are controlled by Cdk1-mediated phosphorylation of Cdc13. Nucleic Acids Res. 37, 3602–3611 (2009).
Grandin, N., Reed, S.I. & Charbonneau, M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11, 512–527 (1997).
Evans, S.K. & Lundblad, V. Est1 and Cdc13 as comediators of telomerase access. Science 286, 117–120 (1999).
Grandin, N., Damon, C. & Charbonneau, M. Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment. Mol. Cell. Biol. 20, 8397–8408 (2000).
Chandra, A., Hughes, T.R., Nugent, C.I. & Lundblad, V. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 15, 404–414 (2001).
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001).
Taggart, A.K., Teng, S.C. & Zakian, V.A. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297, 1023–1026 (2002).
Bianchi, A., Negrini, S. & Shore, D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol. Cell 16, 139–146 (2004).
Puglisi, A., Bianchi, A., Lemmens, L., Damay, P. & Shore, D. Distinct roles for yeast Stn1 in telomere capping and telomerase inhibition. EMBO J. 27, 2328–2339 (2008).
Zhao, X. & Blobel, G.A. SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl. Acad. Sci. USA 102, 4777–4782 (2005).
Potts, P.R. & Yu, H. The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat. Struct. Mol. Biol. 14, 581–590 (2007).
Chen, X.L. et al. Topoisomerase I-dependent viability loss in Saccharomyces cerevisiae mutants defective in both SUMO conjugation and DNA repair. Genetics 177, 17–30 (2007).
Xhemalce, B. et al. Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc. Natl. Acad. Sci. USA 104, 893–898 (2007).
Ungar, L. et al. A genome-wide screen for essential yeast genes that affect telomere length maintenance. Nucleic Acids Res. 37, 3840–3849 (2009).
Rog, O., Miller, K.M., Ferreira, M.G. & Cooper, J.P. SUMOylation of RecQ helicase controls the fate of dysfunctional telomeres. Mol. Cell 33, 559–569 (2009).
Lu, C.Y., Tsai, C.H., Brill, S.J. & Teng, S.C. SUMOylation of the BLM ortholog, Sgs1, promotes telomere-telomere recombination in budding yeast. Nucleic Acids Res. 38, 488–498 (2010).
Johnson, E.S. Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 (2004).
Johnson, E.S. & Gupta, A.A. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106, 735–744 (2001).
Duan, X. et al. Structural and functional insights into the roles of the Mms21 subunit of the Smc5/6 complex. Mol. Cell 35, 657–668 (2009).
Reindle, A. et al. Multiple domains in Siz SUMO ligases contribute to substrate selectivity. J. Cell Sci. 119, 4749–4757 (2006).
Wohlschlegel, J.A., Johnson, E.S., Reed, S.I. & Yates, J.R. III. Global analysis of protein SUMOylation in Saccharomyces cerevisiae. J. Biol. Chem. 279, 45662–45668 (2004).
Fellerhoff, B., Eckardt-Schupp, F. & Friedl, A.A. Subtelomeric repeat amplification is associated with growth at elevated temperature in yku70 mutants of Saccharomyces cerevisiae. Genetics 154, 1039–1051 (2000).
Makovets, S. & Blackburn, E.H. DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nat. Cell Biol. 11, 1383–1386 (2009).
Zhang, W. & Durocher, D. De novo telomere formation is suppressed by the Mec1-dependent inhibition of Cdc13 accumulation at DNA breaks. Genes Dev. 24, 502–515 (2010).
Sacher, M., Pfander, B., Hoege, C. & Jentsch, S. Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat. Cell Biol. 8, 1284–1290 (2006).
Sun, J. et al. Structural bases of dimerization of yeast telomere protein Cdc13 and its interaction with the catalytic subunit of DNA polymerase alpha. Cell Res. 21, 258–274 (2011).
Hughes, T.R., Weilbaecher, R.G., Walterscheid, M. & Lundblad, V. Identification of the single-strand telomeric DNA binding domain of the Saccharomyces cerevisiae Cdc13 protein. Proc. Natl. Acad. Sci. USA 97, 6457–6462 (2000).
Wang, M.J. et al. Telomere-binding and Stn1p-interacting activities are required for the essential function of Saccharomyces cerevisiae Cdc13p. Nucleic Acids Res. 28, 4733–4741 (2000).
Qi, H. & Zakian, V.A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev. 14, 1777–1788 (2000).
Hsu, C.L. et al. Interaction of Saccharomyces Cdc13p with Pol1p, Imp4p, Sir4p and Zds2p is involved in telomere replication, telomere maintenance and cell growth control. Nucleic Acids Res. 32, 511–521 (2004).
Petreaca, R.C. et al. Chromosome end protection plasticity revealed by Stn1p and Ten1p bypass of Cdc13p. Nat. Cell Biol. 8, 748–755 (2006).
Peterson, S.E. et al. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nat. Genet. 27, 64–67 (2001).
Stellwagen, A.E., Haimberger, Z.W., Veatch, J.R. & Gottschling, D.E. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17, 2384–2395 (2003).
Fisher, T.S., Taggart, A.K. & Zakian, V.A. Cell cycle-dependent regulation of yeast telomerase by Ku. Nat. Struct. Mol. Biol. 11, 1198–1205 (2004).
Fisher, T.S. & Zakian, V.A. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amst.) 4, 1215–1226 (2005).
Teo, S.H. & Jackson, S.P. Telomerase subunit overexpression suppresses telomere-specific checkpoint activation in the yeast yku80 mutant. EMBO Rep. 2, 197–202 (2001).
Lustig, A.J. & Petes, T.D. Identification of yeast mutants with altered telomere structure. Proc. Natl. Acad. Sci. USA 83, 1398–1402 (1986).
Hannich, J.T. et al. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280, 4102–4110 (2005).
Makhnevych, T. et al. Global map of SUMO function revealed by protein-protein interaction and genetic networks. Mol. Cell 33, 124–135 (2009).
Ferreira, H.C. et al. The PIAS homologue Siz2 regulates perinuclear telomere position and telomerase activity in budding yeast. Nat. Cell Biol. advance online publication, doi:10.1038/ncb2263 (12 June 2011).
Teixeira, M.T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell 117, 323–335 (2004).
Ghaemmaghami, S. et al. Global analysis of protein expression in yeast. Nature 425, 737–741 (2003).
Tsalik, E.L. & Gartenberg, M.R. Curing Saccharomyces cerevisiae of the 2 micron plasmid by targeted DNA damage. Yeast 14, 847–852 (1998).
Burgess, R.C., Rahman, S., Lisby, M., Rothstein, R. & Zhao, X. The Slx5-Slx8 complex affects SUMOylation of DNA repair proteins and negatively regulates recombination. Mol. Cell Biol. 27, 6153–6162 (2007).
James, P., Halladay, J. & Craig, E.A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 (1996).
Reid, R.J., Lisby, M. & Rothstein, R. Cloning-free genome alterations in Saccharomyces cerevisiae using adaptamer-mediated PCR. Methods Enzymol. 350, 258–277 (2002).
Johnson, E.S. & Blobel, G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J. Cell Biol. 147, 981–994 (1999).
Mao, Y., Sun, M., Desai, S.D. & Liu, L.F. SUMO-1 conjugation to topoisomerase I: A possible repair response to topoisomerase-mediated DNA damage. Proc. Natl. Acad. Sci. USA 97, 4046–4051 (2000).
Takahashi, Y. et al. Cooperation of SUMOylated chromosomal proteins in rDNA maintenance. PLoS Genet. 4, e1000215 (2008).
Palancade, B. et al. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent SUMOylation processes. Mol. Biol. Cell 18, 2912–2923 (2007).
Runge, K.W. & Zakian, V.A. TEL2, an essential gene required for telomere length regulation and telomere position effect in Saccharomyces cerevisiae. Mol. Cell Biol. 16, 3094–3105 (1996).
Roy, N. & Runge, K.W. Two paralogs involved in transcriptional silencing that antagonistically control yeast life span. Curr. Biol. 10, 111–114 (2000).
Zhao, X. & Rothstein, R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc. Natl. Acad. Sci. USA 99, 3746–3751 (2002).
Acknowledgements
We thank S. Li (Duke–National University of Singapore Graduate Medical School) and E. Blackburn (University of California, San Francisco) for the Phos-Thr308 antibody and for several strains, as well as acknowledge S. Li's kind help in using these reagents. We thank M. Lei and S. Gasser for sharing unpublished results; M. Arneric, B. Luke, M. Hohl and A. Chan for helping to set up the G-overhang and telomere measurement experiments; Zhao lab members, particularly C. Cremona and P. Sarangi, and N. Lue and T. Weinert for comments on the manuscript; as well as V.A. Zakian, in whose lab some of this work was carried out, for discussions on experiments and comments on the manuscript. This work was supported by US National Institutes of Health (NIH) grant R01GM080670 and Bressler Scholars Award (to X.Z.), a fellowship from the Canadian Institutes of Health Research (to I. C.) and NIH grant GM43265 to V.A. Zakian.
Author information
Authors and Affiliations
Contributions
X.Z. directed the study. X.Z., X.L. and L.E.H. designed the experiments. X.L., L.E.H., Y.Y. and I.C. carried out the experiments. All authors were involved in data analysis. The manuscript was prepared by X.Z. with the assistance of L.E.H. and X.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 2591 kb)
Rights and permissions
About this article
Cite this article
Hang, L., Liu, X., Cheung, I. et al. SUMOylation regulates telomere length homeostasis by targeting Cdc13. Nat Struct Mol Biol 18, 920–926 (2011). https://doi.org/10.1038/nsmb.2100
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2100
This article is cited by
-
Structural insights into telomere protection and homeostasis regulation by yeast CST complex
Nature Structural & Molecular Biology (2020)
-
Tpz1TPP1 prevents telomerase activation and protects telomeres by modulating the Stn1-Ten1 complex in fission yeast
Communications Biology (2019)
-
Fine tuning the level of the Cdc13 telomere-capping protein for maximal chromosome stability performance
Current Genetics (2019)
-
Cis and trans interactions between genes encoding PAF1 complex and ESCRT machinery components in yeast
Current Genetics (2018)
-
Telomere-end processing: mechanisms and regulation
Chromosoma (2014)