Abstract
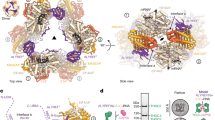
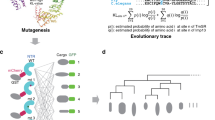
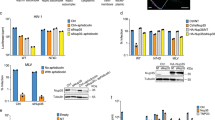
mRNA export is mediated by the TAP–p15 heterodimer, which belongs to the family of NTF2-like export receptors. TAP–p15 heterodimers also bind to the constitutive transport element (CTE) present in simian type D retroviral RNAs, and they mediate the export of viral unspliced RNAs to the host cytoplasm. We have solved the crystal structure of the RNA recognition and leucine-rich repeat motifs of TAP bound to one symmetrical half of the CTE RNA. L-shaped conformations of protein and RNA are involved in a mutual molecular embrace on complex formation. We have monitored the impact of structure-guided mutations on binding affinities in vitro and transport assays in vivo. Our studies define the principles by which CTE RNA subverts the mRNA export receptor TAP, thereby facilitating the nuclear export of viral genomic RNAs, and, more generally, provide insights on cargo RNA recognition by mRNA export receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chook, Y.M. & Blobel, G. Karyopherins and nuclear import. Curr. Opin. Struct. Biol. 11, 703–715 (2001).
Conti, E. & Izaurralde, E. Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol. 13, 310–319 (2001).
Conti, E., Muller, C.W. & Stewart, M. Karyopherin flexibility in nucleocytoplasmic transport. Curr. Opin. Struct. Biol. 16, 237–244 (2006).
Cook, A., Bono, F., Jinek, M. & Conti, E. Structural biology of nucleocytoplasmic transport. Annu. Rev. Biochem. 76, 647–671 (2007).
Köhler, A. & Hurt, E. Exporting RNA from the nucleus to the cytoplasm. Nat. Rev. Mol. Cell Biol. 8, 761–773 (2007).
Stewart, M. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8, 195–208 (2007).
Cook, A.G. & Conti, E. Nuclear export complexes in the frame. Curr. Opin. Struct. Biol. 20, 247–252 (2010).
Cook, A.G., Fukuhara, N., Jinek, M. & Conti, E. Structures of the tRNA export factor in the nuclear and cytosolic states. Nature 461, 60–65 (2009).
Okada, C. et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science 326, 1275–1279 (2009).
Braun, I.C., Rohrbach, E., Schmitt, C. & Izaurralde, E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 18, 1953–1965 (1999).
Grüter, P. et al. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1, 649–659 (1998).
Izaurralde, E. Friedrich Miescher Prize awardee lecture review. A conserved family of nuclear export receptors mediates the exit of messenger RNA to the cytoplasm. Cell. Mol. Life Sci. 58, 1105–1112 (2001).
Segref, A. et al. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 16, 3256–3271 (1997).
Fribourg, S., Braun, I.C., Izaurralde, E. & Conti, E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell 8, 645–656 (2001).
Herold, A. et al. TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 20, 8996–9008 (2000).
Liker, E., Fernandez, E., Izaurralde, E. & Conti, E. The structure of the mRNA export factor TAP reveals a cis arrangement of a non-canonical RNP domain and an LRR domain. EMBO J. 19, 5587–5598 (2000).
Ho, D.N., Coburn, G.A., Kang, Y., Cullen, B.R. & Georgiadis, M.M. The crystal structure and mutational analysis of a novel RNA-binding domain found in the human Tap nuclear mRNA export factor. Proc. Natl. Acad. Sci. USA 99, 1888–1893 (2002).
Grant, R.P., Neuhaus, D. & Stewart, M. Structural basis for the interaction between the Tap/NXF1 UBA domain and FG nucleoporins at 1 resolution. J. Mol. Biol. 326, 849–858 (2003).
Bachi, A. et al. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6, 136–158 (2000).
Ernst, R.K., Bray, M., Rekosh, D. & Hammarskjold, M.L. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol. Cell. Biol. 17, 135–144 (1997).
Tabernero, C., Zolotukhin, A.S., Valentin, A., Pavlakis, G.N. & Felber, B.K. The posttranscriptional control element of the simian retrovirus type 1 forms an extensive RNA secondary structure necessary for its function. J. Virol. 70, 5998–6011 (1996).
Cullen, B.R. Viral RNAs: lessons from the enemy. Cell 136, 592–597 (2009).
Li, Y. et al. An intron with a constitutive transport element is retained in a Tap messenger RNA. Nature 443, 234–237 (2006).
Kang, Y. & Cullen, B.R. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13, 1126–1139 (1999).
Braun, I.C., Herold, A., Rode, M., Conti, E. & Izaurralde, E. Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276, 20536–20543 (2001).
Jin, L., Guzik, B.W., Bor, Y.C., Rekosh, D. & Hammarskjold, M.L. Tap and NXT promote translation of unspliced mRNA. Genes Dev. 17, 3075–3086 (2003).
Serganov, A., Polonskaia, A., Ehresmann, B., Ehresmann, C. & Patel, D.J. Ribosomal protein S15 represses its own translation via adaptation of an rRNA-like fold within its mRNA. EMBO J. 22, 1898–1908 (2003).
Batey, R.T., Rambo, R.P. & Doudna, J.A. Tertiary motifs in RNA structure and folding. Angew. Chem. Int. Edn Engl. 38, 2326–2343 (1999).
Hermann, T. & Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 287, 820–825 (2000).
Tereshko, V., Skripkin, E. & Patel, D.J. Encapsulating streptomycin within a small 40-mer RNA. Chem. Biol. 10, 175–187 (2003).
Dibrov, S.M., Johnston-Cox, H., Weng, Y.H. & Hermann, T. Functional architecture of HCV IRES domain II stabilized by divalent metal ions in the crystal and in solution. Angew. Chem. Int. Edn Engl. 46, 226–229 (2007).
Leontis, N.B. & Westhof, E. Geometric nomenclature and classification of RNA base pairs. RNA 7, 499–512 (2001).
Hermann, T. & Patel, D.J. RNA bulges as architectural and recognition motifs. Structure 8, R47–R54 (2000).
Jiang, F. et al. Anchoring an extended HTLV-1 Rex peptide within an RNA major groove containing junctional base triples. Structure 7, 1461–1472 (1999).
Cléry, A., Blatter, M. & Allain, F.H. RNA recognition motifs: boring? Not quite. Curr. Opin. Struct. Biol. 18, 290–298 (2008).
Lunde, B.M., Moore, C. & Varani, G. RNA-binding proteins: modular design for efficient function. Nat. Rev. Mol. Cell Biol. 8, 479–490 (2007).
Serganov, A. & Patel, D.J. Towards deciphering the principles underlying an mRNA recognition code. Curr. Opin. Struct. Biol. 18, 120–129 (2008).
Price, S.R., Evans, P.R. & Nagai, K. Crystal structure of the spliceosomal U2B-U2A′ protein complex bound to a fragment of U2 small nuclear RNA. Nature 394, 645–650 (1998).
Teplova, M. et al. Structural basis for recognition and sequestration of UUU(OH) 3′ temini of nascent RNA polymerase III transcripts by La, a rheumatic disease autoantigen. Mol. Cell 21, 75–85 (2006).
Liu, L. et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
Hautbergue, G.M., Hung, M.L., Golovanov, A.P., Lian, L.Y. & Wilson, S.A. Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc. Natl. Acad. Sci. USA 105, 5154–5159 (2008).
Stutz, F. & Izaurralde, E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 13, 319–327 (2003).
Walsh, M.J., Hautbergue, G.M. & Wilson, S.A. Structure and function of mRNA export adaptors. Biochem. Soc. Trans. 38, 232–236 (2010).
Huang, Y., Gattoni, R., Stevenin, J. & Steitz, J.A. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell 11, 837–843 (2003).
CCP4. Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Pikovskaya, O., Serganov, A.A., Polonskaia, A., Serganov, A. & Patel, D.J. Preparation and crystallization of riboswitch-ligand complexes. Methods Mol. Biol. 540, 115–128 (2009).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Winn, M.D., Isupov, M.N. & Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 (2001).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 (2005).
Robertson, M.P. & Scott, W.G. A general method for phasing novel complex RNA crystal structures without heavy-atom derivatives. Acta Crystallogr. D Biol. Crystallogr. D64, 738–744 (2008).
Terwilliger, T.C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 (2008).
Huang, X.J. et al. Minimal Rev-response element for type 1 human immunodeficiency virus. J. Virol. 65, 2131–2134 (1991).
Acknowledgements
This research was supported by funds from the US National Institutes of Health (CA049982) to D.J.P. and the Max-Planck Society to E.I. X-ray data were collected at the X-29 beamline at the National Synchrotron Light Source at the Brookhaven National Laboratory and we are grateful to the staff for their assistance.
Author information
Authors and Affiliations
Contributions
Constructs design, protein and RNA preparation and purification, crystallization of complex and its structure determination and in vitro binding assays were undertaken by M.T. with the assistance of N.W.K. under the supervision of D.J.P. The in vivo transport assays were performed by L.W. under the supervision of E.I. The paper was written by M.T., D.J.P. and E.I. with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Methods (PDF 716 kb)
Rights and permissions
About this article
Cite this article
Teplova, M., Wohlbold, L., Khin, N. et al. Structure-function studies of nucleocytoplasmic transport of retroviral genomic RNA by mRNA export factor TAP. Nat Struct Mol Biol 18, 990–998 (2011). https://doi.org/10.1038/nsmb.2094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2094
This article is cited by
-
The nascent RNA binding complex SFiNX licenses piRNA-guided heterochromatin formation
Nature Structural & Molecular Biology (2019)
-
An account of solvent accessibility in protein-RNA recognition
Scientific Reports (2018)
-
Regulation of RNA-binding proteins affinity to export receptors enables the nuclear basket proteins to distinguish and retain aberrant mRNAs
Scientific Reports (2016)
-
Gammaretroviral pol sequences act in cis to direct polysome loading and NXF1/NXT-dependent protein production by gag-encoded RNA
Retrovirology (2014)
-
TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export
Nature Communications (2012)