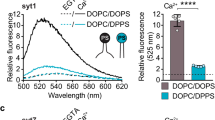
Abstract
Synaptotagmin-1 is a Ca2+ sensor that triggers synchronous neurotransmitter release. The first documented biochemical property of synaptotagmin-1 was its ability to aggregate membranes in response to Ca2+. However, the mechanism and function of this process were poorly understood. Here we show that synaptotagmin-1–mediated vesicle aggregation is driven by trans interactions between synaptotagmin-1 molecules bound to different membranes. We found a strong correlation between the ability of Ca2+-bound synaptotagmin-1 to aggregate vesicles and to stimulate SNARE-mediated membrane fusion. Moreover, artificial aggregation of membranes—using non-synaptotagmin proteins—also efficiently promoted fusion of SNARE-bearing liposomes. Finally, using a modified fusion assay, we observed that synaptotagmin-1 drove the assembly of otherwise non-fusogenic individual t-SNARE proteins into fusion-competent heterodimers, independently of aggregation. Thus, membrane aggregation and t-SNARE assembly appear to be two key aspects of fusion reactions that are regulated by Ca2+-bound synaptotagmin-1 and catalyzed by SNAREs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Koh, T.W. & Bellen, H.J. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 26, 413–422 (2003).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Chicka, M.C., Hui, E., Liu, H. & Chapman, E.R. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat. Struct. Mol. Biol. 15, 827–835 (2008).
Popoli, M. & Mengano, A. A hemagglutinin specific for sialic acids in a rat brain synaptic vesicle-enriched fraction. Neurochem. Res. 13, 63–67 (1988).
Popoli, M., Paterno, R. & Campanella, G. Identification and partial purification of a GM1-binding protein from presynaptic vesicles. Acta Neurol. (Napoli) 13, 213–219 (1991).
Damer, C.K. & Creutz, C.E. Synergistic membrane interactions of the two C2 domains of synaptotagmin. J. Biol. Chem. 269, 31115–31123 (1994).
Damer, C.K. & Creutz, C.E. Calcium-dependent self-association of synaptotagmin I. J. Neurochem. 67, 1661–1668 (1996).
Araç, D. et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Connell, E. et al. Cross-linking of phospholipid membranes is a conserved property of calcium-sensitive synaptotagmins. J. Mol. Biol. 380, 42–50 (2008).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Gaffaney, J.D., Dunning, F.M., Wang, Z., Hui, E. & Chapman, E.R. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283, 31763–31775 (2008).
Diao, J., Yoon, T.Y., Su, Z., Shin, Y.K. & Ha, T. C2AB: a molecular glue for lipid vesicles with a negatively charged surface. Langmuir 25, 7177–7180 (2009).
Garcia, R.A., Forde, C.E. & Godwin, H.A. Calcium triggers an intramolecular association of the C2 domains in synaptotagmin. Proc. Natl. Acad. Sci. USA 97, 5883–5888 (2000).
Ubach, J. et al. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry 40, 5854–5860 (2001).
Wu, Y. et al. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA 100, 2082–2087 (2003).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Bhalla, A., Tucker, W.C. & Chapman, E.R. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell 16, 4755–4764 (2005).
Martens, S., Kozlov, M.M. & McMahon, H.T. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 (2007).
Hui, E., Johnson, C.P., Yao, J., Dunning, F.M. & Chapman, E.R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell 138, 709–721 (2009).
Xue, M., Ma, C., Craig, T.K., Rosenmund, C. & Rizo, J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15, 1160–1168 (2008).
Ohki, S., Duzgunes, N. & Leonards, K. Phospholipid vesicle aggregation: effect of monovalent and divalent ions. Biochemistry 21, 2127–2133 (1982).
Green, N.M. Avidin. 1. The use of (14-C)biotin for kinetic studies and for assay. Biochem. J. 89, 585–591 (1963).
Rufener, E., Frazier, A.A., Wieser, C.M., Hinderliter, A. & Cafiso, D.S. Membrane-bound orientation and position of the synaptotagmin C2B domain determined by site-directed spin labeling. Biochemistry 44, 18–28 (2005).
Hui, E., Bai, J. & Chapman, E.R. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 91, 1767–1777 (2006).
Connor, J. & Schroit, A.J. Determination of lipid asymmetry in human red cells by resonance energy transfer. Biochemistry 26, 5099–5105 (1987).
Bai, J., Wang, P. & Chapman, E.R. C2A activates a cryptic Ca2+-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 99, 1665–1670 (2002).
Shahin, V. et al. Synaptotagmin perturbs the structure of phospholipid bilayers. Biochemistry 47, 2143–2152 (2008).
Wang, P., Wang, C.T., Bai, J., Jackson, M.B. & Chapman, E.R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 278, 47030–47037 (2003).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Leslie, C.C. Properties and regulation of cytosolic phospholipase A2. J. Biol. Chem. 272, 16709–16712 (1997).
Bai, J., Wang, C.T., Richards, D.A., Jackson, M.B. & Chapman, E.R. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron 41, 929–942 (2004).
Lynch, K.L. et al. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol. Biol. Cell 19, 5093–5103 (2008).
Chapman, E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 (2008).
Zhang, X., Kim-Miller, M.J., Fukuda, M., Kowalchyk, J.A. & Martin, T.F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611 (2002).
Stein, A., Radhakrishnan, A., Riedel, D., Fasshauer, D. & Jahn, R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat. Struct. Mol. Biol. 14, 904–911 (2007).
Lynch, K.L. et al. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell 18, 4957–4968 (2007).
Bennett, M.K., Calakos, N. & Scheller, R.H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257, 255–259 (1992).
Söllner, T., Bennett, M.K., Whiteheart, S.W., Scheller, R.H. & Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993).
Chapman, E.R., Hanson, P.I., An, S. & Jahn, R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667–23671 (1995).
Schiavo, G., Stenbeck, G., Rothman, J.E. & Sollner, T.H. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. USA 94, 997–1001 (1997).
Kaetzel, M.A. et al. Phosphorylation mutants elucidate the mechanism of annexin IV-mediated membrane aggregation. Biochemistry 40, 4192–4199 (2001).
Fernández-Alfonso, T., Kwan, R. & Ryan, T.A. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron 51, 179–186 (2006).
Earles, C.A., Bai, J., Wang, P. & Chapman, E.R. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 154, 1117–1123 (2001).
Chen, Y.A., Scales, S.J., Patel, S.M., Doung, Y.C. & Scheller, R.H. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97, 165–174 (1999).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Hui, E., Johnson, C.P., Yao, J., Dunning, F.M. & Chapman, E.R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell 138, 709–721 (2009).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Ubach, J. et al. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry 40, 5854–5860 (2001).
Wu, Y. et al. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA 100, 2082–2087 (2003).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Gaffaney, J.D., Dunning, F.M., Wang, Z., Hui, E. & Chapman, E.R. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283, 31763–31775 (2008).
Ohki, S., Duzgunes, N. & Leonards, K. Phospholipid vesicle aggregation: effect of monovalent and divalent ions. Biochemistry 21, 2127–2133 (1982).
Acknowledgements
We thank members of the Chapman laboratory for their discussions and comments. This work was supported by the Howard Hughes Medical Institute (E.R.C.), the US National Institutes of Health (NIH) National Institute of Mental Health grant MH061876 (to E.R.C.), and the NIH National Institutes on Deafness and Other Communication Disorders grant 1K99DC011267-01 (to C.P.J.).
Author information
Authors and Affiliations
Contributions
E.R.C. conceived of and supervised the project; E.H., J.D.G. and E.R.C. designed the experiments and wrote the manuscript; E.H. conducted all the aggregation assays on synaptotagmin-1 proteins and cPLA2-C2, membrane penetration assays and FRET-based membrane binding assays; J.D.G. conducted all the fusion assays on synaptotagmin-1 proteins and cPLA2-C2 and performed experiments for Figure 6 with Z.W.; Z.W. carried out experiments for Figure 9; C.P.J. conducted avidin-biotin–mediated aggregation and fusion assays in Figure 2d,i and Figure 8e–h; C.S.E. carried out experiments in Supplementary Figure 8.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 1725 kb)
Rights and permissions
About this article
Cite this article
Hui, E., Gaffaney, J., Wang, Z. et al. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat Struct Mol Biol 18, 813–821 (2011). https://doi.org/10.1038/nsmb.2075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2075
This article is cited by
-
Synaptotagmin-7 outperforms synaptotagmin-1 to promote the formation of large, stable fusion pores via robust membrane penetration
Nature Communications (2023)
-
Lysine acetylation regulates the interaction between proteins and membranes
Nature Communications (2021)
-
Function of Drosophila Synaptotagmins in membrane trafficking at synapses
Cellular and Molecular Life Sciences (2021)
-
Synaptotagmin 1 clamps synaptic vesicle fusion in mammalian neurons independent of complexin
Nature Communications (2019)
-
FerA is a Membrane-Associating Four-Helix Bundle Domain in the Ferlin Family of Membrane-Fusion Proteins
Scientific Reports (2018)