Abstract
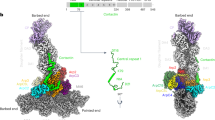
Many actin-binding proteins contain calponin homology (CH) domains, but the manner in which these domains interact with F-actin has been controversial. Crystal structures have shown the tandem CH domains of α-actinin to be in a compact, closed conformation, but the interpretations of complexes of such tandem CH domains with F-actin have been ambiguous. We show that the tandem CH domains of α-actinin bind F-actin in an open conformation, explaining mutations that cause human diseases and suggesting that the opening of these domains may be one of the main regulatory mechanisms for proteins with tandem CH domains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lehman, W., Craig, R., Kendrick-Jones, J. & Sutherland-Smith, A.J. J. Muscle Res. Cell Motil. 25, 351–358 (2004).
Galkin, V.E., Orlova, A., VanLoock, M.S. & Egelman, E.H. J. Mol. Biol. 331, 967–972 (2003).
Broderick, M.J. & Winder, S.J. Adv. Protein Chem. 70, 203–246 (2005).
Lee, S.H., Weins, A., Hayes, D.B., Pollak, M.R. & Dominguez, R. J. Mol. Biol. 376, 317–324 (2008).
Borrego-Diaz, E. et al. J. Struct. Biol. 155, 230–238 (2006).
Keep, N.H. et al. Structure 7, 1539–1546 (1999).
Norwood, F.L., Sutherland-Smith, A.J., Keep, N.H. & Kendrick-Jones, J. Structure 8, 481–491 (2000).
Sawyer, G.M., Clark, A.R., Robertson, S.P. & Sutherland-Smith, A.J. J. Mol. Biol. 390, 1030–1047 (2009).
Liu, J., Taylor, D.W. & Taylor, K.A. J. Mol. Biol. 338, 115–125 (2004).
McGough, A., Way, M. & DeRosier, D. J. Cell Biol. 126, 433–443 (1994).
Hanein, D., Matsudaira, P. & DeRosier, D.J. J. Cell Biol. 139, 387–396 (1997).
Galkin, V.E., Orlova, A., Cherepanova, O., Lebart, M.C. & Egelman, E.H. Proc. Natl. Acad. Sci. USA 105, 1494–1498 (2008).
Kuhlman, P.A., Hemmings, L. & Critchley, D.R. FEBS Lett. 304, 201–206 (1992).
Weins, A. et al. Proc. Natl. Acad. Sci. USA 104, 16080–16085 (2007).
Levine, B.A., Moir, A.J., Patchell, V.B. & Perry, S.V. FEBS Lett. 263, 159–162 (1990).
Corrado, K., Mills, P.L. & Chamberlain, J.S. FEBS Lett. 344, 255–260 (1994).
Way, M., Pope, B. & Weeds, A.G. J. Cell Biol. 119, 835–842 (1992).
Young, P. & Gautel, M. EMBO J. 19, 6331–6340 (2000).
Garcia-Alvarez, B., Bobkov, A., Sonnenberg, A. & de Pereda, J.M. Structure 11, 615–625 (2003).
Galkin, V.E., Orlova, A., Fattoum, A., Walsh, M.P. & Egelman, E.H. J. Mol. Biol. 359, 478–485 (2006).
Acknowledgements
This work was supported by US National Institutes of Health grant GM081303 (to E.H.E.) and by Austrian Science Fund grant P19060 (to K.D.-C.).
Author information
Authors and Affiliations
Contributions
A.S. and A.O. performed sample preparations; A.O. did the electron microscopy; V.E.G. performed the image analysis and model building; V.E.G., A.O., E.H.E. and K.D.-C. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figure 1 (PDF 1785 kb)
Rights and permissions
About this article
Cite this article
Galkin, V., Orlova, A., Salmazo, A. et al. Opening of tandem calponin homology domains regulates their affinity for F-actin. Nat Struct Mol Biol 17, 614–616 (2010). https://doi.org/10.1038/nsmb.1789
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1789
This article is cited by
-
Biased localization of actin binding proteins by actin filament conformation
Nature Communications (2020)
-
Proteins with calmodulin-like domains: structures and functional roles
Cellular and Molecular Life Sciences (2019)
-
Structural basis of the filamin A actin-binding domain interaction with F-actin
Nature Structural & Molecular Biology (2018)
-
Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia
Nature Communications (2018)
-
Structural basis for high-affinity actin binding revealed by a β-III-spectrin SCA5 missense mutation
Nature Communications (2017)