Abstract
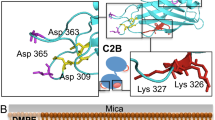
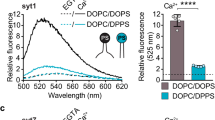
Synchronous neurotransmission is triggered when Ca2+ binds to synaptotagmin 1 (Syt1), a synaptic-vesicle protein that interacts with SNAREs and membranes. We used single-molecule fluorescence resonance energy transfer (FRET) between synaptotagmin's two C2 domains to determine that their conformation consists of multiple states with occasional transitions, consistent with domains in random relative motion. SNARE binding results in narrower intrasynaptotagmin FRET distributions and less frequent transitions between states. We obtained an experimentally determined model of the elusive Syt1–SNARE complex using a multibody docking approach with 34 FRET-derived distances as restraints. The Ca2+-binding loops point away from the SNARE complex, so they may interact with the same membrane. The loop arrangement is similar to that of the crystal structure of SNARE-induced Ca2+-bound Syt3, suggesting a common mechanism by which the interaction between synaptotagmins and SNAREs aids in Ca2+-triggered fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fernandez-Chacon, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Rhee, J.S. et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA 102, 18664–18669 (2005).
Brunger, A.T. Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 38, 1–47 (2005).
Rizo, J. & Rosenmund, C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 15, 665–674 (2008).
Cho, W. & Stahelin, R.V. Membrane binding and subcellular targeting of C2 domains. Biochim. Biophys. Acta 1761, 838–849 (2006).
Pang, Z.P., Shin, O.H., Meyer, A.C., Rosenmund, C. & Sudhof, T.C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 26, 12556–12565 (2006).
Giraudo, C.G. et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science 323, 512–516 (2009).
Maximov, A., Tang, J., Yang, X., Pang, Z.P. & Sudhof, T.C. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323, 516–521 (2009).
Rasnik, I., Myong, S., Cheng, W., Lohman, T.M. & Ha, T. DNA-binding orientation and domain conformation of the E. coli rep helicase monomer bound to a partial duplex junction: single-molecule studies of fluorescently labeled enzymes. J. Mol. Biol. 336, 395–408 (2004).
Wozniak, A.K., Schroder, G.F., Grubmuller, H., Seidel, C.A. & Oesterhelt, F. Single-molecule FRET measures bends and kinks in DNA. Proc. Natl. Acad. Sci. USA 105, 18337–18342 (2008).
Andrecka, J. et al. Single-molecule tracking of mRNA exiting from RNA polymerase II. Proc. Natl. Acad. Sci. USA 105, 135–140 (2008).
Dai, H., Shen, N., Arac, D. & Rizo, J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 367, 848–863 (2007).
Vrljic, M., Strop, P., Ernst, J., Sutton, R.B., Chu, S. & Brunger, A.T. Molecular mechanism of the synaptotagmin–SNARE interaction in Ca2+-triggered vesicle fusion. Nat. Struct. Mol. Biol. advance online publication, doi:10.1038/nsmb.1764 (21 February 2010).
Deniz, A.A., Mukhopadhyay, S. & Lemke, E.A. Single-molecule biophysics: at the interface of biology, physics and chemistry. J. R. Soc. Interface 5, 15–45 (2008).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Shao, X. et al. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron 18, 133–142 (1997).
Shao, X., Fernandez, I., Sudhof, T.C. & Rizo, J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry 37, 16106–16115 (1998).
Ubach, J., Zhang, X., Shao, X., Sudhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Bai, J., Wang, C.T., Richards, D.A., Jackson, M.B. & Chapman, E.R. Fusion pore dynamics are regulated by synaptotagmin*t–SNARE interactions. Neuron 41, 929–942 (2004).
Tang, J. et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 (2006).
Sutton, R.B., Ernst, J.A. & Brunger, A.T. Crystal structure of the cytosolic C2A–C2B domains of synaptotagmin III. Implications for Ca2+-independent SNARE complex interaction. J. Cell Biol. 147, 589–598 (1999).
Fuson, K.L., Montes, M., Robert, J.J. & Sutton, R.B. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry 46, 13041–13048 (2007).
Arac, D. et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Herrick, D.Z. et al. Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: evidence for bilayer bridging. J. Mol. Biol. 390, 913–923 (2009).
Haugland, R.P. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies (Molecular Probes, Carlsbad, California, USA, 2005).
Joo, C. et al. Real-time observation of RecA filament dynamics with single monomer resolution. Cell 126, 515–527 (2006).
Weninger, K., Bowen, M.E., Chu, S. & Brunger, A.T. Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. Proc. Natl. Acad. Sci. USA 100, 14800–14805 (2003).
Bowen, M.E., Weninger, K., Ernst, J., Chu, S. & Brunger, A.T. Single-molecule studies of synaptotagmin and complexin binding to the SNARE complex. Biophys. J. 89, 690–702 (2005).
Muschielok, A. et al. A nano-positioning system for macromolecular structural analysis. Nat. Methods 5, 965–971 (2008).
Majumdar, D.S. et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc. Natl. Acad. Sci. USA 104, 12640–12645 (2007).
Chapman, E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 (2008).
Rickman, C. et al. Conserved prefusion protein assembly in regulated exocytosis. Mol. Biol. Cell 17, 283–294 (2006).
de Wit, H. et al. Synaptotagmin 1 docks secretory vesicles to syntaxin 1–SNAP-25 acceptor complexes. Cell 138, 935–946 (2009).
Xue, M., Ma, C., Craig, T.K., Rosenmund, C. & Rizo, J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15, 1160–1168 (2008).
Tokumaru, H., Shimizu-Okabe, C. & Abe, T. Direct interaction of SNARE complex binding protein synaphin/complexin with calcium sensor synaptotagmin 1. Brain Cell Biol. 36, 173–189 (2008).
Chicka, M.C. & Chapman, E.R. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes (dagger). Biochemistry 48, 657–659 (2009).
Giraudo, C.G. et al. Distinct domains of complexins bind SNARE complexes and clamp fusion in vitro. J. Biol. Chem. 283, 21211–21219 (2008).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 (1998).
Henzler-Wildman, K. & Kern, D. Dynamic personalities of proteins. Nature 450, 964–972 (2007).
Weninger, K., Bowen, M.E., Choi, U.B., Chu, S. & Brunger, A.T. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin–SNAP-25 complex. Structure 16, 308–320 (2008).
Okumus, B., Wilson, T.J., Lilley, D.M.J. & Ha, T. Vesicle encapsulation studies reveal that single molecule ribozyme heterogeneities are intrinsic. Biophys. J. 87, 2798–2806 (2004).
Boukobza, E., Sonnenfeld, A. & Haran, G. Immobilization in surface-tethered lipid vesicles as a new tool for single biomolecule spectroscopy. J. Phys. Chem. B 105, 12165–12170 (2001).
Cisse, I., Okumus, B., Joo, C. & Ha, T.J. Fueling protein-DNA interactions inside porous nanocontainers. Proc. Natl. Acad. Sci. USA 104, 12646–12650 (2007).
Li, Y., Augustine, G.J. & Weninger, K. Kinetics of complexin binding to the SNARE complex: correcting single molecule FRET measurements for hidden events. Biophys. J. 93, 2178–2187 (2007).
Brunger, A.T. Version 1.2 of the Crystallography and NMR System. Nat. Protoc. 2, 2728–2733 (2007).
Acknowledgements
We thank M. Bowen, G. Schröder, T. Südhof and J. Rizo for comments, the US National Institutes of Health for support to A.T.B. (RO1-MH63105) and the Burroughs Wellcome Fund for a CASI award to K.R.W. A.T.B. presented this work at the first Paul B. Sigler lecture at Yale University on 23 March 2009.
Author information
Authors and Affiliations
Contributions
U.B.C. prepared all samples and performed all experimental measurements; P.S., M.V. and A.T.B. performed all simulations; all authors contributed to formulation of the experimental design, interpretation of results and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 1 and 2 and Supplementary Note (PDF 2898 kb)
Rights and permissions
About this article
Cite this article
Choi, U., Strop, P., Vrljic, M. et al. Single-molecule FRET–derived model of the synaptotagmin 1–SNARE fusion complex. Nat Struct Mol Biol 17, 318–324 (2010). https://doi.org/10.1038/nsmb.1763
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1763
This article is cited by
-
Reliability and accuracy of single-molecule FRET studies for characterization of structural dynamics and distances in proteins
Nature Methods (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
miR-128 as a Regulator of Synaptic Properties in 5xFAD Mice Hippocampal Neurons
Journal of Molecular Neuroscience (2021)
-
Selected tools to visualize membrane interactions
European Biophysics Journal (2021)
-
Synaptotagmin 1 clamps synaptic vesicle fusion in mammalian neurons independent of complexin
Nature Communications (2019)