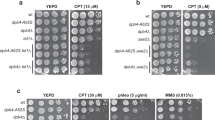
Abstract
ATP-dependent chromatin remodeling complexes have been shown to participate in DNA replication in addition to transcription and DNA repair. However, the mechanisms of their involvement in DNA replication remain unclear. Here, we reveal a specific function of the yeast INO80 chromatin remodeling complex in the DNA damage tolerance pathways. Whereas INO80 is necessary for the resumption of replication at forks stalled by methyl methane sulfonate (MMS), it is not required for replication fork collapse after treatment with hydroxyurea (HU). Mechanistically, INO80 regulates DNA damage tolerance during replication through modulation of PCNA (proliferating cell nuclear antigen) ubiquitination and Rad51-mediated processing of recombination intermediates at impeded replication forks. Our findings establish a mechanistic link between INO80 and DNA damage tolerance pathways, indicating that chromatin remodeling is important for accurate DNA replication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Falbo, K.B. & Shen, X. Chromatin remodeling in DNA replication. J. Cell. Biochem. 97, 684–689 (2006).
Vincent, J.A., Kwong, T.J. & Tsukiyama, T. ATP-dependent chromatin remodeling shapes the DNA replication landscape. Nat. Struct. Mol. Biol. 15, 477–484 (2008).
Papamichos-Chronakis, M. & Peterson, C.L. The Ino80 chromatin-remodeling enzyme regulates replisome function and stability. Nat. Struct. Mol. Biol. 15, 338–345 (2008).
Shimada, K. et al. Ino80 chromatin remodeling complex promotes recovery of stalled replication forks. Curr. Biol. 18, 566–575 (2008).
Shen, X., Mizuguchi, G., Hamiche, A. & Wu, C. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406, 541–544 (2000).
Morrison, A.J. et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119, 767–775 (2004).
Morrison, A.J. et al. Mec1/Tel1 phosphorylation of the INO80 chromatin remodeling complex influences DNA damage checkpoint responses. Cell 130, 499–511 (2007).
van Attikum, H., Fritsch, O., Hohn, B. & Gasser, S.M. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 119, 777–788 (2004).
Katou, Y. et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424, 1078–1083 (2003).
Michalet, X. et al. Dynamic molecular combing: stretching the whole human genome for high-resolution studies. Science 277, 1518–1523 (1997).
Shen, X., Ranallo, R., Choi, E. & Wu, C. Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol. Cell 12, 147–155 (2003).
Tourrière, H., Versini, G., Cordon-Preciado, V., Alabert, C. & Pasero, P. Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol. Cell 19, 699–706 (2005).
Branzei, D. & Foiani, M. The Rad53 signal transduction pathway: Replication fork stabilization, DNA repair, and adaptation. Exp. Cell Res. 312, 2654–2659 (2006).
Branzei, D. & Foiani, M. Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amst.) 6, 994–1003 (2007).
Branzei, D. & Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 9, 297–308 (2008).
Lopes, M. et al. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412, 557–561 (2001).
Rogakou, E.P., Pilch, D.R., Orr, A.H., Ivanova, V.S. & Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Luke, B. et al. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 16, 786–792 (2006).
Branzei, D. et al. Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127, 509–522 (2006).
Hoege, C., Pfander, B., Moldovan, G.L., Pyrowolakis, G. & Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 (2002).
Ulrich, H.D. Conservation of DNA damage tolerance pathways from yeast to humans. Biochem. Soc. Trans. 35, 1334–1337 (2007).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005).
Watts, F.Z. Sumoylation of PCNA: Wrestling with recombination at stalled replication forks. DNA Repair (Amst.) 5, 399–403 (2006).
Chang, M., Bellaoui, M., Boone, C. & Brown, G.W. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA 99, 16934–16939 (2002).
Veis, J., Klug, H., Koranda, M. & Ammerer, G. Activation of the G2/M-specific gene CLB2 requires multiple cell cycle signals. Mol. Cell. Biol. 27, 8364–8373 (2007).
Stelter, P. & Ulrich, H.D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 (2003).
Kao, C.F. & Osley, M.A. In vivo assays to study histone ubiquitylation. Methods 31, 59–66 (2003).
Han, J., Zhou, H., Li, Z., Xu, R.M. & Zhang, Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J. Biol. Chem. 282, 28587–28596 (2007).
Gangavarapu, V., Prakash, S. & Prakash, L. Requirement of RAD52 group genes for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 27, 7758–7764 (2007).
Duro, E., Vaisica, J.A., Brown, G.W. & Rouse, J. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair (Amst.) 7, 811–818 (2008).
Li, X. & Heyer, W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 18, 99–113 (2008).
Branzei, D., Vanoli, F. & Foiani, M. SUMOylation regulates Rad18-mediated template switch. Nature 456, 915–920 (2008).
Tsukuda, T., Fleming, A.B., Nickoloff, J.A. & Osley, M.A. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature 438, 379–383 (2005).
Fasullo, M., Giallanza, P., Dong, Z., Cera, C. & Bennett, T. Saccharomyces cerevisiae rad51 mutants are defective in DNA damage-associated sister chromatid exchanges but exhibit increased rates of homology-directed translocations. Genetics 158, 959–972 (2001).
Pasero, P., Bensimon, A. & Schwob, E. Single-molecule analysis reveals clustering and epigenetic regulation of replication origins at the yeast rDNA locus. Genes Dev. 16, 2479–2484 (2002).
Cobb, J.A., Bjergbaek, L., Shimada, K., Frei, C. & Gasser, S.M. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 22, 4325–4336 (2003).
Acknowledgements
We thank E. Schwob (IGMM) and J. Rouse (MRC) for strains; H. Ulrich (Cancer UK) for strains and protocols; M.A. Osley (University of New Mexico) for protocols; P. Sung (Yale University) for Rad51 antibody; J. Delrow and R. Basom (Fred Hutchinson Cancer Research Center, FHCRC) for microarray analysis; K. Claypool and S. Hasley (M.D. Anderson Cancer Center (MDACC)) for technical assistance; and members of the Shen lab for comments on the manuscript. This work was supported by funds and grants from MDACC, ACS (RSG-05-060-01-GMC) and the National Institute of Environmental Health Sciences (ES07784) to X.S. (MDACC), from the Rosalie B. Hite Fellowship to K.B.F. (MDACC), from the Fondation Recherche Medicale, the Centre National de la Recherche Scientifique, the Agence Nationale de la Recherche and the Institut National du Cancer to P.P.; by an ARC fellowship to C.A. (CNRS); and by a grant from the US National Institutes of Health (GM71729) to Z.Z. K.S. was supported by a grant of the Cell Innovation Project and Grant-in-Aid for Scientific Research (S) from the Ministry of Education Science and Sports (MEXT), Japan. Y.K. is a Global Center of Excellence (GCOE) research associate.
Author information
Authors and Affiliations
Contributions
K.B.F. and X.S. designed the study with contributions from X.H., S.W. and Y.S.; K.B.F., C.A., P.P., Y.K., K.S., J.H. and Z.Z. carried out experiments; T.W. and J.X. provided technical assistance. K.B.F. and X.S. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1 and 2 (PDF 1946 kb)
Rights and permissions
About this article
Cite this article
Falbo, K., Alabert, C., Katou, Y. et al. Involvement of a chromatin remodeling complex in damage tolerance during DNA replication. Nat Struct Mol Biol 16, 1167–1172 (2009). https://doi.org/10.1038/nsmb.1686
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1686
This article is cited by
-
Identification of Nanog as a novel inhibitor of Rad51
Cell Death & Disease (2022)
-
A Novel Cell-Penetrating Antibody Fragment Inhibits the DNA Repair Protein RAD51
Scientific Reports (2019)
-
INO80 exchanges H2A.Z for H2A by translocating on DNA proximal to histone dimers
Nature Communications (2017)
-
ATM-mediated phosphorylation of the chromatin remodeling enzyme BRG1 modulates DNA double-strand break repair
Oncogene (2015)
-
Identification of novel DNA-damage tolerance genes reveals regulation of translesion DNA synthesis by nucleophosmin
Nature Communications (2014)