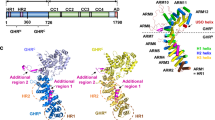
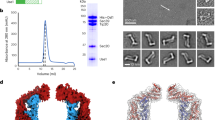
Abstract
Multisubunit tethering complexes are essential for intracellular trafficking and have been proposed to mediate the initial interaction between vesicles and the membranes with which they fuse. Here we report initial structural characterization of the Dsl1p complex, whose three subunits are essential for trafficking from the Golgi apparatus to the endoplasmic reticulum (ER). Crystal structures reveal that two of the three subunits, Tip20p and Dsl1p, resemble known subunits of the exocyst complex, establishing a structural connection among several multisubunit tethering complexes and implying that many of their subunits are derived from a common progenitor. We show, moreover, that Tip20p and Dsl1p interact directly via N-terminal α-helices. Finally, we establish that different Dsl1p complex subunits bind independently to different ER SNARE proteins. Our results map out two alternative protein-interaction networks capable of tethering COPI-coated vesicles, via the Dsl1p complex, to ER membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pfeffer, S.R. Unsolved mysteries in membrane traffic. Annu. Rev. Biochem. 76, 629–645 (2007).
Grosshans, B.L., Ortiz, D. & Novick, P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 103, 11821–11827 (2006).
Pfeffer, S.R. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1, E17–E22 (1999).
Cai, H., Reinisch, K. & Ferro-Novick, S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 12, 671–682 (2007).
Gillingham, A.K. & Munro, S. Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta 1641, 71–85 (2003).
Whyte, J.R. & Munro, S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637 (2002).
Sztul, E. & Lupashin, V. Role of tethering factors in secretory membrane traffic. Am. J. Physiol. Cell Physiol. 290, C11–C26 (2006).
Sato, T.K., Rehling, P., Peterson, M.R., Emr, S.D. & Class, C. Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell 6, 661–671 (2000).
Seals, D.F., Eitzen, G., Margolis, N., Wickner, W.T. & Price, A.A. Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA 97, 9402–9407 (2000).
Kraynack,, B.A. et al. Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol. Biol. Cell 16, 3963–3977 (2005).
Shestakova, A., Suvorova, E., Pavliv, O., Khaidakova, G. & Lupashin, V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J. Cell Biol. 179, 1179–1192 (2007).
Cai, H. et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature 445, 941–944 (2007).
Sacher, M. et al. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 17, 2494–2503 (1998).
Kim, Y.G. et al. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 127, 817–830 (2006).
Terbush, D.R., Maurice, T., Roth, D. & Novick, P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483–6494 (1996).
Ungar, D. et al. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J. Cell Biol. 157, 405–415 (2002).
Dong, G., Hutagalung, A.H., Fu, C., Novick, P. & Reinisch, K.M. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat. Struct. Mol. Biol. 12, 1094–1100 (2005).
Hamburger, Z.A., Hamburger, A.E., West, A.P., Jr & Weis, W.I. Crystal structure of the S. cerevisiae exocyst component Exo70p. J. Mol. Biol. 356, 9–21 (2006).
Moore, B.A., Robinson, H.H. & Xu, Z. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J. Mol. Biol. 371, 410–421 (2007).
Sivaram, M.V., Furgason, M.L., Brewer, D.N. & Munson, M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat. Struct. Mol. Biol. 13, 555–556 (2006).
Wu, S., Mehta, S.Q., Pichaud, F., Bellen, H.J. & Quiocho, F.A. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol. 12, 879–885 (2005).
Cavanaugh, L.F. et al. Structural analysis of conserved oligomeric Golgi complex subunit 2. J. Biol. Chem. 282, 23418–23426 (2007).
Koumandou, V.L., Dacks, J.B., Coulson, R.M. & Field, M.C. Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol. Biol. 7, 29 (2007).
Whyte, J.R. & Munro, S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 1, 527–537 (2001).
Andag, U., Neumann, T. & Schmitt, H.D. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J. Biol. Chem. 276, 39150–39160 (2001).
Reilly, B.A., Kraynack, B.A., VanRheenen, S.M. & Waters, M.G. Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol. Biol. Cell 12, 3783–3796 (2001).
Sweet, D.J. & Pelham, H.R. The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J. 12, 2831–2840 (1993).
VanRheenen, S.M., Reilly, B.A., Chamberlain, S.J. & Waters, M.G. Dsl1p, an essential protein required for membrane traffic at the endoplasmic reticulum/Golgi interface in yeast. Traffic 2, 212–231 (2001).
Kamena, F. & Spang, A. Tip20p prohibits back-fusion of COPII vesicles with the endoplasmic reticulum. Science 304, 286–289 (2004).
Andag, U. & Schmitt, H.D. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J. Biol. Chem. 278, 51722–51734 (2003).
Frigerio, G. The Saccharomyces cerevisiae early secretion mutant tip20 is synthetic lethal with mutants in yeast coatomer and the SNARE proteins Sec22p and Ufe1p. Yeast 14, 633–646 (1998).
Mnaimneh, S. et al. Exploration of essential gene functions via titratable promoter alleles. Cell 118, 31–44 (2004).
Sweet, D.J. & Pelham, H.R. The Saccharomyces cerevisiae SEC20 gene encodes a membrane glycoprotein which is sorted by the HDEL retrieval system. EMBO J. 11, 423–432 (1992).
Novick, P., Ferro, S. & Schekman, R. Order of events in the yeast secretory pathway. Cell 25, 461–469 (1981).
Burri, L. et al. A SNARE required for retrograde transport to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 100, 9873–9877 (2003).
Dilcher, M. et al. Use1p is a yeast SNARE protein required for retrograde traffic to the ER. EMBO J. 22, 3664–3674 (2003).
Pashkova, N., Jin, Y., Ramaswamy, S. & Weisman, L.S. Structural basis for myosin V discrimination between distinct cargoes. EMBO J. 25, 693–700 (2006).
Schott, D., Ho, J., Pruyne, D. & Bretscher, A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147, 791–808 (1999).
Sivaram, M.V., Saporita, J.A., Furgason, M.L., Boettcher, A.J. & Munson, M. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry 44, 6302–6311 (2005).
Munson, M. & Novick, P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 13, 577–581 (2006).
Zhang, X. et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 180, 145–158 (2008).
Ungar, D., Oka, T., Krieger, M. & Hughson, F.M. Retrograde transport on the COG railway. Trends Cell Biol. 16, 113–120 (2006).
Scheich, C., Kummel, D., Soumailakakis, D., Heinemann, U. & Bussow, K. Vectors for co-expression of an unrestricted number of proteins. Nucleic Acids Res. 35, e43 (2007).
Doublié, S. Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 276, 523–530 (1997).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D Biol. Crystallogr. 59, 2023–2030 (2003).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Brunger, A.T. et al. Crystallography & NMR System (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Lovell, S.C. et al. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 50, 437–450 (2003).
Murshudov, G.N. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Storoni, L.C., McCoy, A.J. & Read, R.J. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 60, 432–438 (2004).
Holm, L. & Park, J. DaliLite workbench for protein structure comparison. Bioinformatics 16, 566–567 (2000).
Acknowledgements
We gratefully acknowledge B. Kokona and R. Fairman for sedimentation velocity analytical ultracentrifugation; the staff of the National Synchrotron Light Source X25 and X29 beamlines for assistance with X-ray data collection; O. Perisic for advice on crystallization; M. Diefenbacher and A. Spang for many fruitful discussions and for communicating results before publication; K. Büssow (Max Planck Institute for Molecular Genetics, Berlin) for reagents; and S. Munro, M. Munson, A. Spang and members of the Hughson laboratory for critical comments on the manuscript. This work was supported by the US National Institutes of Health grant GM071574.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 928 kb)
Rights and permissions
About this article
Cite this article
Tripathi, A., Ren, Y., Jeffrey, P. et al. Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol 16, 114–123 (2009). https://doi.org/10.1038/nsmb.1548
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1548
This article is cited by
-
Mechanisms of SNARE proteins in membrane fusion
Nature Reviews Molecular Cell Biology (2024)
-
Sec3 promotes the initial binary t-SNARE complex assembly and membrane fusion
Nature Communications (2017)
-
Crystal structure of Sec10, a subunit of the exocyst complex
Scientific Reports (2017)
-
Molecular architecture of the complete COG tethering complex
Nature Structural & Molecular Biology (2016)
-
Chaperoning SNARE assembly and disassembly
Nature Reviews Molecular Cell Biology (2016)