Abstract
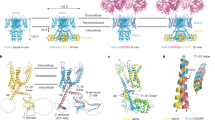
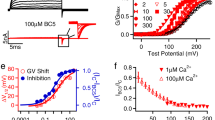
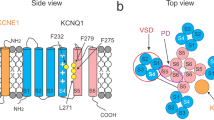
An important step to understanding ion channels is identifying the structural components that act as the gates to ion movement. Here we describe a new channel gating mechanism, produced by the β3 auxiliary subunits of Ca2+-activated, large-conductance BK-type K+ channels when expressed with their pore-forming α subunits. BK β subunits have a cysteine-rich extracellular segment connecting two transmembrane segments, with small cytosolic N and C termini. The extracellular segments of the β3 subunits form gates to block ion permeation, providing a mechanism by which current can be rapidly diminished upon cellular repolarization. Furthermore, this gating mechanism is abolished by reduction of extracellular disulfide linkages, suggesting that endogenous mechanisms may regulate this gating behavior. The results indicate that auxiliary β subunits of BK channels reside sufficiently close to the ion permeation pathway defined by the α subunits to influence or block access of small molecules to the permeation pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
MacKinnon, R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350, 232–235 (1991).
Li, M., Unwin, N., Stauffer, K.A., Jan, Y.N. & Jan, L.Y. Images of purified Shaker potassium channels. Curr. Biol. 4, 110–115 (1994).
Pfaffinger, P.J. & DeRubeis, D. Shaker K+ channel T1 domain self-tetramerizes to a stable structure. J. Biol. Chem. 270, 28595–28600 (1995).
Adelman, J.P. et al. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9, 209–216 (1992).
Butler, A., Tsunoda, S., McCobb, D.P., Wei, A. & Salkoff, L. mSlo, a complex mouse gene encoding 'maxi' calcium-activated potassium channels. Science 261, 221–224 (1993).
Knaus, H.G. et al. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 269, 17274–17278 (1994).
Wallner, M., Meera, P. & Toro, L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. USA 96, 4137–4142 (1999).
Xia, X.M., Ding, J.P. & Lingle, C.J. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19, 5255–5264 (1999).
Xia, X.-M., Ding, J., Zeng, X.-H., Duan, K.-L. & Lingle, C. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J. Neurosci. 20, 4890–4903 (2000).
Uebele, V.N. et al. Cloning and functional expression of two families of β-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 275, 23211–23218 (2000).
Brenner, R., Jegla, T.J., Wickenden, A., Liu, Y. & Aldrich, R.W. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275, 6453–6461 (2000).
McManus, O.B. et al. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron 14, 645–650 (1995).
Jin, P., Weiger, T.M. & Levitan, I.B. Reciprocal modulation between the α and β4 subunits of hSlo calcium-dependent potassium channels. J. Biol. Chem. 277, 43724–43729 (2002).
Meera, P., Wallner, M. & Toro, L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 97, 5562–5567 (2000).
Hanner, M. et al. The β subunit of the high conductance calcium-activated potassium channel. Identification of residues involved in charybdotoxin binding. J. Biol. Chem. 273, 16289–16296 (1998).
Knaus, H.G., Garcia-Calvo, M., Kaczorowski, G.J. & Garcia, M.L. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J. Biol. Chem. 269, 3921–3924 (1994).
Wang, Y.-W., Ding, J.P., Xia, X.-M. & Lingle, C.J. Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of BK-type Ca2+-activated K+ channels. J. Neurosci. 22, 1550–1561 (2002).
Hanner, M. et al. The β subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc. Natl. Acad. Sci. USA 94, 2853–2858 (1997).
Meera, P., Wallner, M. & Toro, L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 97, 5562–5567 (2000).
Zeng, X.-H., Xia, X.-M. & Lingle, C.J. Gating properties conferred on BK channels by the β3b auxiliary subunit in the absence of its N- and C-termini. J. Gen. Physiol. 117, 607–627 (2001).
Cox, D.H., Cui, J. & Aldrich, R.W. Separation of gating properties from permeation and block in mSlo large conductance Ca-activated K+ channels. J. Gen. Physiol. 109, 633–646 (1997).
Lopatin, A.N., Makhina, E.N. & Nichols, C.G. The mechanism of inward rectification of potassium channels: 'long-pore plugging' by cytoplasmic polyamines. J. Gen. Physiol. 106, 923–955 (1995).
Shyng, S.L., Sha, Q., Ferrigni, T., Lopatin, A.N. & Nichols, C.G. Depletion of intracellular polyamines relieves inward rectification of potassium channels. Proc. Natl. Acad. Sci. USA 93, 12014–12019 (1996).
Ding, J.P., Li, Z.W. & Lingle, C.J. Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and noninactivating subunits. Biophys. J. 74, 268–289 (1998).
MacKinnon, R., Aldrich, R.W. & Lee, A.W. Functional stoichiometry of Shaker potassium channel inactivation. Science 262, 757–759 (1993).
Yellen, G. The voltage-gated potassium channels and their relatives. Nature 419, 35–42 (2002).
Bruening-Wright, A., Schumacher, M.A., Adelman, J.P. & Maylie, J. Localization of the activation gate for small conductance Ca2+-activated K+ channels. J. Neurosci. 22, 6499–6506 (2002).
del Camino, D. & Yellen, G. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron 32, 649–656 (2001).
Zhou, M., Morais-Cabral, J.H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001).
Hoshi, T., Zagotta, W.N. & Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation [see comments]. Science 250, 533–538 (1990).
Rettig, J. et al. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature 369, 289–294 (1994).
Armstrong, C.E. & Roberts, W.M. Rapidly inactivating and non-inactivating calcium-activated potassium currents in frog saccular hair cells. J. Physiol. 536, 49–65 (2001).
Weiger, T.M. et al. A novel nervous system β subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 20, 3563–3570 (2000).
Horrigan, F. & Aldrich, R. Coupling between voltage-sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120, 267–305 (2002).
Lingle, C.J. Setting the stage for molecular dissection of the regulatory components of BK channels. J. Gen. Physiol. 120, 261–265 (2002).
Xia, X.M. et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395, 503–507 (1998).
Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981).
Zhang, X., Solaro, C. & Lingle, C. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a non-selective, low affinity divalent cation site. J. Gen. Physiol. 118, 607–635 (2001).
Solaro, C.R., Ding, J.P., Li, Z.W. & Lingle, C.J. The cytosolic inactivation domains of BKi channels in rat chromaffin cells do not behave like simple, open-channel blockers. Biophys. J. 73, 819–830 (1997).
Wallner, M., Meera, P. & Toro, L. Determinant for β-subunit regulation in high-conductance voltage- activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc. Natl. Acad. Sci. USA 93, 14922–14927 (1996).
Acknowledgements
We thank the Department of Anesthesiology, Washington University School of Medicine for material support. X.Z. was supported by an AHA-Missouri affiliate fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zeng, XH., Xia, XM. & Lingle, C. Redox-sensitive extracellular gates formed by auxiliary β subunits of calcium-activated potassium channels. Nat Struct Mol Biol 10, 448–454 (2003). https://doi.org/10.1038/nsb932
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb932
This article is cited by
-
Mapping the functional expression of auxiliary subunits of KCa1.1 in glioblastoma
Scientific Reports (2022)
-
Inter-α/β subunits coupling mediating pre-inactivation and augmented activation of BKCa(β2)
Scientific Reports (2013)
-
Stereospecific binding of a disordered peptide segment mediates BK channel inactivation
Nature (2012)
-
The BK potassium channel in the vascular smooth muscle and kidney: α- and β-subunits
Kidney International (2010)
-
Nitric oxide activates TRP channels by cysteine S-nitrosylation
Nature Chemical Biology (2006)