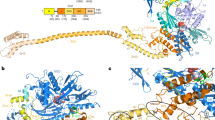
Abstract
In Escherichia coli, the pseudouridine synthase RsuA catalyzes formation of pseudouridine (ψ) at position 516 in 16S rRNA during assembly of the 30S ribosomal subunit. We have determined the crystal structure of RsuA bound to uracil at 2.0 Å resolution and to uridine 5′-monophosphate (UMP) at 2.65 Å resolution. RsuA consists of an N-terminal domain connected by an extended linker to the central and C-terminal domains. Uracil and UMP bind in a cleft between the central and C-terminal domains near the catalytic residue Asp 102. The N-terminal domain shows structural similarity to the ribosomal protein S4. Despite only 15% amino acid identity, the other two domains are structurally similar to those of the tRNA-specific ψ-synthase TruA, including the position of the catalytic Asp. Our results suggest that all four families of pseudouridine synthases share the same fold of their catalytic domain(s) and uracil-binding site.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Charette, M. & Gray, M.W. IUBMB Life 49, 341–351 (2000).
Ban, N., Nissen, P., Hansen, J., Moore, P.B. & Steitz, T.A. Science 289, 905–920 (2000).
Wimberly, B.T. et al. Nature 407, 327–339 (2000).
Bakin, A., Kowalak, J.A., McCloskey, J.A. & Ofengand, J. Nucleic Acids Res. 22, 3681–3684 (1994).
Conrad, J., Niu, L., Rudd, K., Lane, B.G. & Ofengand, J. RNA 5, 751–763 (1999).
Wrzesinski, J., Bakin, A., Nurse, K., Lane, B.G. & Ofengand, J. Biochemistry 34, 8904–8913 (1995).
Ofengand, J., Bakin, A., Wrzesinski, J., Nurse, K. & Lane, B.G. Biochem. Cell Biol. 73, 915–924 (1995).
Koonin, E.V. Nucleic Acids Res. 24, 2411–2415 (1996).
Gustafsson, C., Reid, R., Greene, P.J. & Santi, D.V. Nucleic Acids Res. 24, 3756–3762 (1996).
Huang, L., Pookanjanatavip, M., Gu, X. & Santi, D.V. Biochemistry 37, 344–351 (1998).
Ramamurthy, V., Swann, S.L., Paulson, J.L., Spedaliere, C.J. & Mueller, E.G. J. Biol. Chem. 274, 22225–22230 (1999).
Del Campo, M., Kaya, Y. & Ofengand, J. RNA 7, 1603–1615 (2001).
Foster, P.G., Huang, L., Santi, D.V. & Stroud, R.M. Nature Struct. Biol. 7, 23–27 (2000).
Holm, L. & Sander, C. J. Mol. Biol. 233, 123–138 (1993).
Staker, B.L., Korber, P., Bardwell, J.C. & Saper, M.A. EMBO J. 19, 749–757 (2000).
Davies, C., Gerstner, R.B., Draper, D.E., Ramakrishnan, V. & White, S.W. EMBO J. 17, 4545–4558 (1998).
Sankaranarayanan, R. et al. Cell 97, 371–381 (1999).
Kissinger, C.R., Sieker, L.C., Adman, E.T. & Jensen, L.H. J. Mol. Biol. 219, 693–715 (1991).
Gallagher, D.T. et al. Structure 6, 465–475 (1998).
Rosenzweig, A.C. et al. Structure Fold. Des. 7, 605–617 (1999).
Lindahl,M. et al. EMBO J. 13, 1249–1254 (1994).
Kohls, D., Sulea, T., Purisima, E.O., MacKenzie, R.E. & Vrielink, A. Structure Fold. Des. 8, 35–46 (2000).
Dym, O., Pratt, E.A., Ho, C. & Eisenberg, D. Proc. Natl. Acad. Sci. USA 97, 9413–9418 (2000).
Gu, X., Liu, Y. & Santi, D.V. Proc. Natl. Acad. Sci. USA 96, 14270–14275 (1999).
Chen, J. & Patton, J.R. RNA 5, 409–419 (1999).
Aravind, L. & Koonin, E.V. J. Mol. Evol. 48, 291–302 (1999).
Hoang, C. & Ferre-D'Amare, A.R. Cell 107, 929–939 (2001).
Hendrickson, W.A., Horton, J.R. & LeMaster, D.M. EMBO J. 9, 1665–1672 (1990).
Otwinowski, Z. & Minor,W. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C. & Berendzen, J. Acta Crystallogr. D 55, 849–861 (1999).
Terwilliger, T.C. Acta Crystallogr. D 56, 965–972 (2000).
Jones, T.A., Zhou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
Esnouf, R.M. Acta Crystallogr. D 55, 938–940 (1999).
Thompson, J.D., Higgins, D.G. & Gibson, T.J. Nucleic Acids Res. 22, 4673–4680 (1994).
Acknowledgements
We wish to thank L. Flaks, beamline X8C, NSLS, for assistance with data collection and S. Raymond for computational expertise.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sivaraman, J., Sauvé, V., Larocque, R. et al. Structure of the 16S rRNA pseudouridine synthase RsuA bound to uracil and UMP. Nat Struct Mol Biol 9, 353–358 (2002). https://doi.org/10.1038/nsb788
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb788