Abstract
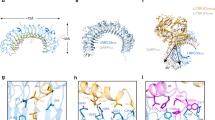
Cysteine-rich repeats in the integrin β subunit stalk region relay activation signals to the ligand-binding headpiece. The NMR solution structure and disulfide bond connectivity of Cys-rich module-3 of the integrin β2 subunit reveal a nosecone-shaped variant of the EGF fold, termed an integrin-EGF (I-EGF) domain. Interdomain contacts between I-EGF domains 2 and 3 observed by NMR support a model in which the modules are related by an approximate two-fold screw axis in an extended arrangement. Our findings complement a 3.1 Å crystal structure of the extracellular portion of integrin αVβ3, which lacks an atomic model for I-EGF2 and a portion of I-EGF3. The disulfide connectivity of I-EGF3 chemically assigned here differs from the pairings suggested in the αVβ3 structure. Epitopes that become exposed upon integrin activation and residues that restrain activation are defined in β2 I-EGF domains 2 and 3. Superposition on the αVβ3 structure reveals that they are buried. This observation suggests that the highly bent αVβ3 structure represents the inactive conformation and that release of contacts with I-EGF modules 2 and 3 triggers a switchblade-like opening motion extending the integrin into its active conformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Humphries, M.J. Biochem. Soc. Trans. 28, 311–339 (2000).
Takagi, J., Erickson, H.P. & Springer, T.A. Nature Struct. Biol. 8, 412–416 (2001).
Lu, C., Takagi, J. & Springer, T.A. J. Biol. Chem. 276, 14642–14648 (2001).
Lu, C., Ferzly, M., Takagi, J. & Springer, T.A. J. Immunol. 166, 5629–5637 (2001).
Zang, Q. & Springer, T.A. J. Biol. Chem. 276, 6922–6929 (2001).
Huang, C., Lu, C. & Springer, T.A. Proc. Natl. Acad. Sci. USA 94, 3156–3161 (1997).
Emsley, J., Knight, C.G., Farndale, R.W., Barnes, M.J. & Liddington, R.C. Cell 101, 47–56 (2000).
Xiong, J.-P. et al. Science 294, 339–345 (2001).
Takagi, J., Beglova, N., Yalamanchili, P., Blacklow, S.C. & Springer, T.A. Proc. Natl. Acad. Sci. USA 98, 11175–11180 (2001).
Tan, S.-M. et al. FEBS Lett. 505, 27–30 (2001).
Bork, P., Downing, A.K., Kieffer, B. & Campbell, I.D. Q. Rev. Biophys. 29, 119–167 (1996).
Rao, Z. et al. Cell 82, 131–141 (1995).
Downing, A.K. et al. Cell 85, 597–605 (1996).
Shih, D.T., Edelman, J.M., Horwitz, A.F., Grunwald, G.B. & Buck, C.A. J. Cell Biol. 122, 1361–1371 (1993).
Faull, R.J. et al. J. Biol. Chem. 271, 25099–25106 (1996).
Bazzoni, G., Shih, D.-T., Buck, C.A. & Hemler, M.A. J. Biol. Chem. 270, 25570–25577 (1995).
Takagi, J., Isobe, T., Takada, Y. & Saito, Y. J. Biochem. (Tokyo) 121, 914–921 (1997).
Du, X. et al. J. Biol. Chem. 268, 23087–23092 (1993).
Lee, B. & Richards, F.M. J. Mol. Biol. 55, 379–400 (1971).
Bailey, S. Acta Crystallogr. D 50, 760–763 (1994).
Janin, J. Nature. Struct. Biol. 4, 973–974 (1997).
Mehta, R.J. et al. Biochem. J. 330, 861–869 (1998).
Smith, J.W., Piotrowicz, R.S. & Mathis, D. J. Biol. Chem. 269, 960–967 (1994).
Hughes, P.E., O'Toole, T.E., Ylanne, J., Shattil, S.J. & Ginsberg, M.H. J. Biol. Chem. 270, 12411–12417 (1995).
Huang, C., Zang, Q., Takagi, J. & Springer, T.A. J. Biol. Chem. 275, 21514–21524 (2000).
Carr, C.M. & Kim, P.S. Cell 73, 823–832 (1993).
Bullough, P.A., Hughson, F.M., Skehel, J.J. & Wiley, D.C. Nature 371, 37–43 (1994).
Andrew, D. et al. Eur. J. Immunol. 23, 2217–2222 (1993).
Studier, F.W. & Moffatt, B.A. J. Mol. Biol. 189, 113–130 (1986).
Blacklow, S.C. & Kim, P.S. Nature Struct. Biol. 3, 758–762 (1996).
North, C.L. & Blacklow, S.C. Biochemistry 38, 3926–3935 (1999).
Cavanagh, J., Palmer, I.A.G., Fairbrother, W. & Skelton, N.J. Protein NMR Spectroscopy: Principles and Practice. (Academic Press, San Diego; 1996).
Pons, J.-L. Malliavin, T.E. & Delsuc, M.A. J. Biomol. NMR 8, 445–452 (1996).
Bai, Y., Milne, J.S., Mayne, L. & Englander, S.W. Proteins 17, 75–86 (1993).
Brunger, A.T. et al. Acta Crystallgr. D 54, 905–921 (1998).
Stickle, D.F., Presta, L.G., Dill, K.A. & Rose, G.D. J. Mol. Biol. 226, 1143–1159 (1992).
Sali, A. & Blundell, T.L. J. Mol. Biol. 234, 779–815 (1993).
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. J. Biomol. NMR 8, 477–486 (1996).
Carson, M. Methods Enzymol. 277, 493–505 (1997).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins 11, 281–296 (1991).
Acknowledgements
Supported by grants from the NIH, Pew Charitable Trust and the American Heart Association.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Beglova, N., Blacklow, S., Takagi, J. et al. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Mol Biol 9, 282–287 (2002). https://doi.org/10.1038/nsb779
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb779
This article is cited by
-
Anoikis resistance––protagonists of breast cancer cells survive and metastasize after ECM detachment
Cell Communication and Signaling (2023)
-
Integrins and their potential roles in mammalian pregnancy
Journal of Animal Science and Biotechnology (2023)
-
The role of integrins in inflammation and angiogenesis
Pediatric Research (2021)
-
Targeting Leukocyte Trafficking in Inflammatory Bowel Disease
BioDrugs (2021)
-
Structural and mechanical functions of integrins
Biophysical Reviews (2014)