Abstract
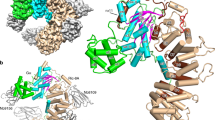
The Rho-related small GTP-binding protein Cdc42 has a low intrinsic GTPase activity that is significantly enhanced by its specific GTPase-activating protein, Cdc42GAP. In this report, we present the tertiary structure for the aluminum fluoride-promoted complex between Cdc42 and a catalytically active domain of Cdc42GAP as well as the complex between Cdc42 and the catalytically compromised Cdc42GAP(R305A) mutant. These structures, which mimic the transition state for the GTP hydrolytic reaction, show the presence of an AlF 3 molecule, as was seen for the corresponding Ras–p120RasGAP complex, but in contrast to what has been reported for the Rho–Cdc42GAP complex or for heterotrimeric G protein α subunits, where AlF 4 – was observed. The Cdc42GAP stabilizes both the switch I and switch II domains of Cdc42 and contributes a highly conserved arginine (Arg 305) to the active site. Comparison of the structures for the wild type and mutant Cdc42GAP complexes provides important insights into the GAP-catalyzed GTP hydrolytic reaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mittal, R., Ahmadian, M.R., Goody, R. & Wittinghofer, F. Science 273, 115–117 ( 1996).
Hoffman, G.R., Nassar, N., Oswald, R. & Cerione, R. J. Biol. Chem . 273, 4392–4399 ( 1998).
Lancaster, C.A. et al. J. Biol. Chem. 269, 1137– 1142 (1994).
Barford, E.T. et al. J. Biol. Chem. 268, 26059– 26062 (1993).
Kjeldgaard, M., Nyborg, J. & Clark, B.F.C. FASEB J. 10, 1347– 1368 (1996).
Wei, Y. et al. Nature Struct. Biol. 4, 699– 703 (1997).
Rittinger, K. et al. Nature 388, 693–697 (1997).
Rittinger, K., Walker, P.A., Eccleston, J.F., Smerdon, S. & Gamblin, S. Nature 389, 758–762 (1997).
Hirshberg M., Stockly R.W., Dodson G. & Webb M. Nature Struct. Biol . 4, 147–151 ( 1997).
Feltham J.L. et al. Biochemistry 36, 8755– 8766 (1997).
Sondeck, J., Lambright, D., Noel, J., Hamm, H. E. & Sigler, P. Nature 372, 276– 279 (1994).
Coleman, D. et al. Science 265, 1405– 1412 (1994).
Scheffzek, K. Science 277, 333–338 ( 1997).
Maegly, K.A., Admiraal, S. & Herschlag, D. Proc. Natl. Acad. Sci. USA 93, 8160–8166 (1996).
Noel, J.B., Hamm, H.E. & Sigler, P. Nature 366, 654– 663 (1993).
Leonard, D.A., Lin, R., Cerione, R. A. & Manor, D. J. Biol. Chem. 273, 16210–16215 (1998).
Zheng, Y., Bagrodia, S. & Cerione, R. J. Biol. Chem. 269, 18727– 18730 (1994).
Sprang S. Science 277, 329–330 ( 1997).
Tesmer, J.G., Berman, D.M., Gilman, A.G. & Sprang, S. R. Cell 89, 251–261 ( 1997).
Hendrickson, W.A., Horton, J.R. & LeMaster, D.M. EMBO J. 9, 1665– 1672 (1990).
Kabsch, W. J. Appl. Crystallogr. 21, 916–924 (1988).
Otwinowski, Z. & Minor, W. Meth. Enz. 276, 307–326 (1997).
Leslie, A.G.W., Brick, P. & Wonacott, A.J. CCP4 News 18, 33– 39 (1986).
CCP4. Acta Crystallogr. D 50, 760–763 (1994).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Read, R.J. Acta Crystallogr. A 42, 140–149 (1986).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 ( 1991).
Laskowski, R.A., McArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Lee, B. & Richards, F.M. J. Mol. Biol. 55, 379–400 (1971).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E.A. and Murphy, M.E.P. Acta Crystallogr. D 50, 869–873 (1994).
Acknowledgements
We thank S. Doublie for invaluable help and comments on this project. We thank R. Sweet for beamtime on X12C and the CHESS staff for help during data collection. We thank J. Stamos for excellent technical assistance, W. Wang for help and C. Westmiller for expert secretarial assistance. This work was supported by grants from the National Institutes of Health and the Human Frontiers Science Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nassar, N., Hoffman, G., Manor, D. et al. Structures of Cdc42 bound to the active and catalytically compromised forms of Cdc42GAP. Nat Struct Mol Biol 5, 1047–1052 (1998). https://doi.org/10.1038/4156
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/4156
This article is cited by
-
Patient-derived mutations within the N-terminal domains of p85α impact PTEN or Rab5 binding and regulation
Scientific Reports (2018)
-
Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network
Nature Genetics (2015)
-
Structural analyses of Legionella LepB reveal a new GAP fold that catalytically mimics eukaryotic RasGAP
Cell Research (2013)
-
Recognition of the F&H motif by the Lowe syndrome protein OCRL
Nature Structural & Molecular Biology (2011)
-
The Rap–RapGAP complex: GTP hydrolysis without catalytic glutamine and arginine residues
The EMBO Journal (2008)