Abstract
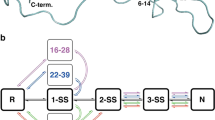
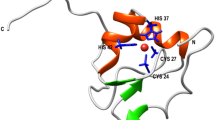
Bovine pancreatic trypsin inhibitor (BPTI) does not fold by simple sequential formation of its native disulphide bonds. Instead, an initially formed intermediate, termed N', first rearranges to a more stable species in a slow process that requires substantial unfolding. We find that direct oxidation of N' is also inhibited by native structure which slows both the intermolecular step in oxidation—formation of a mixed disulphide bond with the oxidizing agent GSSG—as well as the subsequent intramolecular step. Folding does not occur appreciably by direct oxidation because the high GSSG concentrations required for efficient mixed disulphide formation cause N' to accumulate as a nonproductive, double-mixed disulphide species. The need to unfold previously acquired native structure, observed in the folding of BPTI, may be a common feature of disulphide-linked folding reactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Goldenberg, D.P. Native and non-native intermediates in the BPTI folding pathway. Trends Biochem. Sci. 17, 257–261 (1992).
Creighton, T.E. Conformational restrictions on the pathway of folding and unfolding of the pancreatic trypsin inhibitor. J. molec. Biol. 113, 275–293 (1977).
Creighton, T.E. & Goldenberg, D.P. Kinetic role of a meta-stable native-like two-disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J. molec. Biol. 179, 497–526 (1984).
Weissman, J.S. & Kim, P.S. Reexamination of the folding of BPTI: predominance of native intermediates. Science 253, 1386–1393 (1991).
Weissman, J.S. & Kim, P.S. Kinetic role of nonnative species in the folding of bovine pancreatic trypsin inhibitor. Proc. natn. Acad. Sci. U.S.A. 89, 9900–9904 (1992).
Dadlez, M. & Kim, P.S. Detection of a third native one-disulfide intermediate in the folding of BPTI. Nature struct. Biol., 2, 674–679 (1995).
Weissman, J.S. & Kim, P.S. The pro region of BPTI facilitates folding. Cell 71, 841–851 (1992).
Weissman, J.S. & Kim, P.S. Efficient catalysis of disulfide bond rearrangements by protein disulfide isomerase. Nature 365, 185–188 (1993).
Creighton, T.E., Bagley, C.J., Cooper, L., Darby, N.J., Freedman, R.B., Kemmink, J. & Sheikh, A. On the biosynthesis of bovine pancreatic trypsin inhibitor (BPTI). J. molec. Biol. 232, 1176–1196 (1993).
Moses, E. & Hinz, H.-J. Basic pancreatic trypsin inhibitor has unusual thermodynamic stability parameters. J. molec. Biol. 170, 765–776 (1983).
Makhatadze, G.I., Kim, K.-S., Woodward, C. & Privalov, P.L. Thermodynamics of BPTI folding. Prot. Sci. 2, 2028–2036 (1993).
States, D.J., Dobson, C.M., Karplus, M. & Creighton, T.E. A new two-disulphide intermediate in the refolding of reduced bovine pancreatic trypsin inhibitor. J. molec. Biol. 174, 411–418 (1984).
Eigenbrot, C., Randal, M. & Kossiakoff, A.A. Structural effects induced by removal of a disulfide-bridge: the X-ray structure of the C30A/C51A mutant of basic pancreatic trypsin inhibitor. Prot. Engng. 3, 591–598 (1990).
van Mierlo, C.P., Darby, N.J., Neuhaus, D. & Creighton, T.E. (14–38, 30–51) double-disulphide intermediate in folding of bovine pancreatic trypsin inhibitor: a two-dimensional 1H nuclear magnetic resonance study. J. molec. Biol. 222, 353–371 (1991).
Schulman, B.A. & Kim, P.S. Hydrogen exchange in BPTI variants that do not share a common disulfide bond. Prot. Sci. 3, 2226–2232 (1994).
Darby, N.J., Morin, P.E., Talbo, G. & Creighton, T.E. Refolding of bovine pancreatic trypsin inhibitor via non-native disulphide intermediates. J. molec. Biol. 249, 463–477 (1995).
Stassinopoulou, C.I., Wagner, G. & Wüthrich, K. Two-dimensional 1H NMR of two chemically modified analogs of the basic pancreatic trypsin inhibitor. Eur. J. Biochem. 145, 423–430 (1984).
Mendoza, J.A., Jarstfer, M.B. & Goldenberg, D.P. Effects of amino acid replacements on the reductive unfolding kinetics of pancreatic trypsin inhibitor. Biochemistry 33, 1143–1148 (1994).
Altman, J.D., Henner, D., Nilsson, B., Anderson, S. & Kuntz, I.D. Intracellular expression of BPTI fusion proteins and single column cleavage/affinity purification by chymotrypsin. Prot. Engng. 4, 593–600 (1991).
Hvidt, A. & Nielson, S.O. Hydrogen exchange in proteins. Adv. Prot. Chem. 21, 287–386 (1966).
Lee, B. & Richards, F.M. The interpretation of protein structures: estimation of static accessibility. J. molec. Biol. 55, 379–400 (1971).
Deisenhofer, J. & Steigemann, W. Crystallographic refinement of the structure of bovine pancreatic trypsin inhibitor at 1.5Å resolution. Acta Crystallogr. B31, 238–250 (1975).
Wlodawer, A., Nachman, J., Gilliland, G.L., Gallagher, W. & Woodward, C. Structure of form III crystals of bovine pancreatic trypsin inhibitor. J. molec. Biol. 198, 469–480 (1987).
Kikuchi, H., Goto, Y. & Hamaguchi, K. Reduction of the buried intrachain disulphide bond of the constant fragment of the immunoglobulin light chain: global unfolding under physiological conditions. Biochemistry 25, 2009–2013 (1986).
Oas, T.G. & Kim, P.S. A peptide model of a protein folding intermediate. Nature 336, 42–48 (1988).
van Mierlo, C.P., Darby, N.J. & Creighton, T.E. The partially folded conformation of the [30–51] intermediate in the disulfide folding pathway of bovine pancreatic trypsin inhibitor. Proc. natn. Acad. Sci. U.S.A. 89, 6775–6779 (1992).
Staley, J.P. & Kim, P.S. Formation of a native-like subdomain in a partially folded intermediate of bovine pancreatic trypsin inhibitor. Prot. Sci. 3, 1822–1832 (1994).
Page, M.I. & Jencks, W.P. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and chelate effect. Proc. natn. Acad. Sci. U.S.A. 68, 1678–1683 (1971).
Creighton, T.E. An empirical approach to protein conformation stability and flexibility. Biopolymers 22, 49–58 (1983).
Lin, T.-Y. & Kim, P.S. Urea dependence of thiol-disulfide equilibria in thioredoxin: confirmation of the linkage relationship and a sensitive assay for structure. Biochemistry 28, 5282–5287 (1989).
Rothwarf, D.M. & Scheraga, H.A. Regeneration of bovine pancreatic ribonuclease A. 4. temperature dependence of the regeneration rate. Biochemistry 32, 2698–2703 (1993).
Marks, C.B., Naderi, H., Kosen, P.A., Kuntz, I.D. & Anderson, S. Mutants of bovine pancreatic trypsin inhibitor lacking cysteines 14 and 38 can fold properly. Science 235, 1370–1373 (1987).
Pace, N.C., Grimsley, G.R., Thomson, J.A. & Barnett, B.J. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J. biol. Chem. 263, 11820–11825 (1988).
Goto, Y. & Hamaguchi, K. The role of the intrachain disulphide bond in the conformation and stability of the constant fragment of the immunoglobulin light chain. J. Biochem. 86, 1433–1441 (1979).
Mucke, M. & Schmid, F.X. Intact disulfide bonds decelerate the folding of ribonuclease T1. J. molec. Biol. 239, 713–725 (1994).
Srinivasan, N., Sowdhamini, R., Ramakrishnan, C. & Balaram, P. Conformations of disulfide bridges in proteins. Int. J. Peptide Res. 36, 147–155 (1990).
Bardwell, J.C., McGovern, K. & Beckwith, J. Identification of a protein required for disulphide bond formation in vivo. Cell 67, 581–590 (1991).
Wunderlich, M., Otto, A., Seckler, R. & Glockshuber, R. Bacterial protein disulfide isomerase: efficient catalysis of oxidative protein folding at acidic pH. Biochemistry 32, 12251–12260 (1993).
Zapun, A. & Creighton, T.E. Effects of DsbA on the disulfide folding of bovine pancreatic trypsin inhibitor and α-lactalbumin. Biochemistry 33, 5202–5211 (1994).
Ellman, G. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 82, 70–77 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weissman, J., Kim, P. A kinetic explanation for the rearrangement pathway of BPTI folding. Nat Struct Mol Biol 2, 1123–1130 (1995). https://doi.org/10.1038/nsb1295-1123
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1295-1123