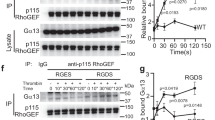
Abstract
Integrin α4β7 mediates rolling adhesion in Ca2+ and Ca2+ + Mg2+, and firm adhesion in Mg2+ and Mn2+, mimicking the two key steps in leukocyte accumulation in inflamed vasculature. We mutated an interlinked linear array of three divalent cation-binding sites present in integrin β-subunit I-like domains. The middle, metal ion–dependent adhesion site (MIDAS) is required for both rolling and firm adhesion. One polar site, that adjacent to MIDAS (ADMIDAS), is required for rolling because its mutation results in firm adhesion. The other polar site, the ligand-induced metal binding site (LIMBS), is required for firm adhesion because its mutation results in rolling. The LIMBS mediates the positive regulatory effects of low Ca2+ concentrations, whereas the ADMIDAS mediates the negative regulatory effects of higher Ca2+ concentrations, which are competed by Mn2+. The bipolar sites thus stabilize two alternative phases of adhesion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Shimaoka, M., Takagi, J. & Springer, T.A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 (2002).
Emsley, J., Knight, C.G., Farndale, R.W., Barnes, M.J. & Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 101, 47–56 (2000).
Beglova, N., Blacklow, S.C., Takagi, J. & Springer, T.A. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 9, 282–287 (2002).
Shimaoka, M. et al. Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 (2003).
Takagi, J., Petre, B.M., Walz, T. & Springer, T.A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599–611 (2002).
Takagi, J., Strokovich, K., Springer, T.A. & Walz, T. Structure of integrin α5β1 in complex with fibronectin. EMBO J. 22, 4607–4615 (2003).
Xiong, J.-P. et al. Crystal structure of the extracellular segment of integrin αVβ3. Science 294, 339–345 (2001).
Xiong, J.P. et al. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 (2002).
Marlin, S.D. & Springer, T.A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51, 813–819 (1987).
Gailit, J. & Ruoslahti, E. Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 263, 12927–12932 (1988).
Dransfield, I., Cabañas, C., Craig, A. & Hogg, N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 116, 219–226 (1992).
Staatz, W.D., Rajpara, S.M., Wayner, E.A., Carter, W.G. & Santoro, S.A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J. Cell Biol. 108, 1917–1924 (1989).
Mould, A.P., Akiyama, S.K. & Humphries, M.J. Regulation of integrin α5β1-fibronectin interactions by divalent cations. J. Biol. Chem. 270, 26270–26277 (1995).
Hu, D.D., Hoyer, J.R. & Smith, J.W. Ca2+ suppresses cell adhesion to osteopontin by attenuating binding affinity for integrin αvβ3. J. Biol. Chem. 270, 9917–9925 (1995).
Leitinger, B., McDowall, A., Stanley, P. & Hogg, N. The regulation of integrin function by Ca2+. Biochim. Biophys. Acta 1498, 91–98 (2000).
Berlin, C. et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422 (1995).
Alon, R. et al. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 128, 1243–1253 (1995).
de Chateau, M., Chen, S., Salas, A. & Springer, T.A. Kinetic and mechanical basis of rolling through an integrin and novel Ca2+-dependent rolling and Mg2+-dependent firm adhesion modalities for the α4β7–MAdCAM-1 interaction. Biochemistry 40, 13972–13979 (2001).
Springer, T.A. Predicted and experimental structures of integrins and β-propellers. Curr. Opin. Struct. Biol. 12, 802–813 (2002).
Pujades, C. et al. Defining extracellular integrin α chain sites that affect cell adhesion and adhesion strengthening without altering soluble ligand binding. Mol. Biol. Cell 8, 2647–2657 (1997).
Knorr, R. & Dustin, M.L. The lymphocyte function-associated antigen 1 I domain is a transient binding module for intercellular adhesion molecule (ICAM)-1 and ICAM-1 in hydrodynamic flow. J. Exp. Med. 186, 719–730 (1997).
Lu, C., Shimaoka, M., Zang, Q., Takagi, J. & Springer, T.A. Locking in alternate conformations of the integrin αLβ2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc. Natl. Acad. Sci. USA 98, 2393–2398 (2001).
Salas, A., Shimaoka, M., Chen, S., Carman, C.V. & Springer, T.A. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin LFA-1. J. Biol. Chem. 277, 50255–50262 (2002).
Berlin, C. et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993).
Bargatze, R.F., Jutila, M.A. & Butcher, E.C. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 3, 99–108 (1995).
Briskin, M.J., McEvoy, L.M. & Butcher, E.C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature 363, 461–464 (1993).
Chang, K.-C., Tees, D.F. & Hammer, D.A. The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. USA 97, 11262–11267 (2000).
Harding, M.M. Geometry of metal-ligand interactions in proteins. Acta Crystallogr. D 57, 401–411 (2001).
Tidswell, M. et al. Structure-function analysis of the integrin β7 subunit: Identification of domains involved in adhesion to MAdCAM-1. J. Immunol. 159, 1497–1505 (1997).
Lu, C. & Springer, T.A. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1 (LFA-1). J. Immunol. 159, 268–278 (1997).
Lu, C., Oxvig, C. & Springer, T.A. The structure of the β-propeller domain and C-terminal region of the integrin αM subunit. J. Biol. Chem. 273, 15138–15147 (1998).
Kassner, P.D. & Hemler, M.E. Interchangeable α chain cytoplasmic domains play a positive role in control of cell adhesion mediated by VLA-4, a β1 integrin. J. Exp. Med. 178, 649–660 (1993).
Lazarovits, A.I. et al. Lymphocyte activation antigens: I. A monoclonal antibody, anti-act I, defines a new late lymphocyte activation antigen. J. Immunol. 133, 1857–1862 (1984).
Schweighoffer, T. et al. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J. Immunol. 151, 717–729 (1993).
Lawrence, M.B. & Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65, 859–873 (1991).
Acknowledgements
We thank D.J. Erle and M.J. Briskin for providing wild-type human integrin β7 cDNA and α4β7 K562 stable transfectant and human MAdCAM-1/Fc, respectively. This work was supported by a grant from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chen, J., Salas, A. & Springer, T. Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat Struct Mol Biol 10, 995–1001 (2003). https://doi.org/10.1038/nsb1011
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb1011
This article is cited by
-
A mutation that blocks integrin α4β7 activation prevents adaptive immune-mediated colitis without increasing susceptibility to innate colitis
BMC Biology (2020)
-
TLR3 agonists induce fibronectin aggregation by activated astrocytes: a role of pro-inflammatory cytokines and fibronectin splice variants
Scientific Reports (2020)
-
Immunoregulation of macrophages by dynamic ligand presentation via ligand–cation coordination
Nature Communications (2019)
-
General structural features that regulate integrin affinity revealed by atypical αVβ8
Nature Communications (2019)
-
Relationship of cardiovascular disease risk factors and noncoding RNAs with hypertension: a case-control study
BMC Cardiovascular Disorders (2018)