Abstract
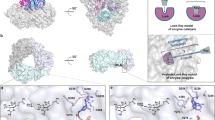
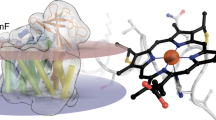
Heme oxygenase catalyzes the first step in the oxidative degradation of heme. The crystal structure of heme oxygenase-1 (HO-1) reported here reveals a novel helical fold with the heme sandwiched between two helices. The proximal helix provides a heme iron ligand, His 25. Conserved glycines in the distal helix near the oxygen binding site allow close contact between the helix backbone and heme in addition to providing flexibility for substrate binding and product release. Regioselective oxygenation of the α-meso heme carbon is due primarily to steric influence of the distal helix.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Tenhunen, R., Marver, H.S. & Schmid, R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 244, 6388–6394 (1969).
Dore, S. et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 96, 2445–2450 (1999).
Verma, A., Hirsch, D.J., Glatt, C.E., Ronnett, G.V. & Snyder, S.H. Carbon monoxide: a putative neural messenger. Science 259, 381–384 (1993).
Maines, M.D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554 (1997).
Mueller, R.M., Taguchi, H. & Shibahara, S. Nucleotide sequence and organization of the rat heme oxygenase gene. J. Biol. Chem. 262, 6795–6802 (1987).
Yoshida, T., Biro, P., Cohen, T., Mueller, R.M. & Shibahara, S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur. J. Biochem. 171, 457–461 (1988).
Yachie, A. et al. Oxidative stress causes enhanced endothelial injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 103, 129–135 (1998).
Poss, K.D. & Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA 94, 10919–10924 (1997).
Hancock, W.W., Buelow, R., Sayegh, M.H. & Turka, L.A. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat. Med. 4, 1392–1396 (1998).
Soares, M.P. et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat. Med. 4, 1073–1077 (1998).
Rotenberg, M.O. & Maines, M.D. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J. Biol. Chem. 265, 7501–7506 (1990).
Zakhary, R. et al. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. USA 94, 14848–14853 (1997).
Zhuo, M., Small, S.A., Kandel, E.R. & Hawkins, R.D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science 260, 1946–1950 (1993).
Schmitt, M.P. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179, 838–845 (1997).
Muramoto, T., Kohchi, T., Yokota, A., Hwang, I. & Goodman, H.M. The Arabidopsis photomorphogenic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell 11, 335–347 (1999).
Cornejo, J., Willows, R.D. & Beale, S.I. Phytobilin biosynthesis: cloning and expression of a gene encoding soluble ferredoxin-dependent heme oxygenase in Synechocystis sp. PCC 6803. Plant J. 15, 99–107 (1998).
Yoshida, T. & Sato, M. Posttranslational and direct integration of heme oxygenase into microsomes. Biochem. Biophys. Res. Commun. 163, 1086–1092 (1989).
Ortiz de Montellano, P.R. Heme oxygenase mechanism: evidence for an electrophilic ferric peroxide species. Acc. Chem. Res. 31, 543–549 (1998).
Takahashi, S. et al. Heme-heme oxygenase complex. Structure of the catalytic site and its implication for oxygen activation. J. Biol. Chem. 269, 1010–1014 (1994).
Sun, J., Wilks, A., Ortiz de Montellano, P.R. & Loehr, T.M. Resonance Raman and EPR spectroscopic studies on heme-heme oxygenase complexes. Biochemistry 32, 14151–14157 (1993).
Sano, S., Sano, T., Morishima, I., Shiro, Y. & Maeda, Y. On the mechanism of the chemical and enzymatic oxygenation of α oxyprotohemin IX to Fe-biliverdin Xα. Proc. Natl. Acad. Sci. USA 83, 531–535 (1986).
Migita, C.T. et al. The oxygen and carbon monoxide reactions of heme oxygenase. J. Biol. Chem. 273, 945–949 (1998).
Yoshida, T., Ishikawa, K. & Sato, M. Degradation of heme by a soluble peptide of heme oxygenase obtained from rat liver microsomes by mild trypsinization. Eur. J. Biochem. 199, 729–733 (1991).
Ishikawa, K., Sato, M., Ito, M. & Yoshida, T. Importance of histidine residue 25 of rat heme oxygenase for its catalytic activity. Biochem. Biophys. Res. Commun. 182, 981–986 (1992).
Wilks, A., Black, S.M., Miller, W.L. & Ortiz de Montellano, P.R. Expression and characterization of truncated human heme oxygenase hHO-1 and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry 34, 4421–4427 (1995).
Wilks, A., Medzihradszky, K.F. & Ortiz de Montellano, P.R. Heme oxygenase active-site residues identified by heme-protein cross-linking during reduction of CBrCl3. Biochemistry 37, 2889–2896 (1998).
Schuller, D.J., Wilks, A., Ortiz de Montellano, P.R. & Poulos, T.L. Crystallization of recombinant human heme oxygenase-1. Protein Sci. 7, 1836–1838 (1998).
Omata, Y., Asada, S., Sakamoto, H., Fukuyama, K. & Noguchi, M. Crystallization and preliminary X-ray diffraction studies on the water soluble form of rat heme oxygenase-1 in complex with heme. Acta Crystallogr. D 54 1017–1019 (1998).
Wang, M. et al. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. USA 94, 8411–8416 (1997).
Sevrioukova, I.F., Li, H., Zhang, H., Peterson, J.A. & Poulos, T.L. Structure of a cytochrome P450-redox partner electron transfer complex. Proc. Natl. Acad. Sci. USA 96, 1863–1868 (1999).
Wilks, A., Sun, J., Loehr, T.M. & Ortiz de Montellano, P.R. Heme oxygenase His25Ala mutant: replacement of the proximal histidine iron ligand by exogenous bases restores catalytic activity. J. Am. Chem. Soc. 117, 2925–2926 (1995).
Frydman, R.B. et al. Specificity of heme oxygenase: a study with synthetic hemins. Biochemistry 20, 5177–5182 (1981).
Hernandez, G. et al. Proton NMR investigation of substrate-bound heme oxygenase: evidence for electronic and steric contributions to stereoselective heme cleavage. Biochemistry 33, 6631–6641 (1994).
Takahashi, S. et al. Heme-heme oxygenase complex: structure and properties of the ccatalytic site form resonance Raman scattering. Biochemistry 33, 5531–5538 (1994).
Collman, J.P. et al. O2 and CO binding to iron (II) porphyrins: a comparison of the "picket fence" and "pocket" porphyrins. J. Am. Chem. Soc. 105, 3052–3064 (1983).
Traylor, T.G., Koga, N. & Dearduff, L.A. Structural differentiation of CO and O2 binding to iron porphyrins: polar pocket effects. J. Am. Chem. Soc. 107, 6504–6510 (1985).
Lavalette, D., Tetreau, C., Mispelter, J., Momenteau, M. & Lhoste, J.-M. Linear free-energy relationships in binding of oxygen and carbon monoxide with heme model compounds and heme proteins. Eur. J. Biochem. 145, 555–565 (1984).
Springer, B.A., Sligar, S.G., Olson, J.S. & Phillips, G.N. Mechanisms of ligand recognition in myoglobin. Chem. Rev. 94, 669–714 (1994).
Kachalova, G.S., Popov, A.N. & Bartunk, H.D. A steric mechanism for inhibition of CO binding to heme proteins. Science 284, 473–476 (1999).
Traylor, T.G., Koga, N., Dearduff, L.A., Sweptson, P.N. & Ibers, J.A. 1,3,-adamantane-3,13-porphyrin-6,6-cyclophane: crystal structure of the free base and steric effects on ligation of the iron (II) complex. J. Am. Chem. Soc. 106, 5132–5143 (1984).
Ghosh, A. & Bocian, D.F. The carbonyl tilting and bending potential surface of carbon monoxyhemes. J. Phys. Chem. 100, 6363–6367 (1996).
Sigfridsson, E. & Ryde, U. On the significance of hydrogen bonds for the discrimination between CO and O2 by myoglobin. J. Biol. Inorg. Chem. 4, 99–110 (1999).
Murakami, T. et al. Effects of the arrangement of a distal catalytic residue on regioselectivity and reactivity in the coupled oxidation of sperm whale myoglobin mutants. J. Am. Chem. Soc. 121, 2007–2011 (1999).
Brown, S.B., Chabot, A.A., Enderby, E.A. & North, A.C.T. Orientation of oxygen in oxyhaemoproteins and its implications for haem catabolism. Nature 289, 93–95 (1981).
Noguchi, M., Yoshida, T. & Kikuchi, G. A stoichiometric study of heme degradation catalyzed by the reconstituted heme oxygenase system with special consideration of the production of hydrogen peroxide during the reaction. J. Biochem. 93, 1027–1036 (1983).
Rodríquez, J.C. & Rivera, M. Conversion of mitochondrial cytochrome b5 into a species capable of performing the efficient coupled oxidation of heme. Biochemistry 37, 13082–13090 (1998).
Torpey, J. & Ortiz de Montellano, P.R. Oxidation of the meso-methylmesoheme regioisomers by heme oxygenase. J. Biol. Chem. 271, 26067–26073 (1996).
Torpey, J. & Ortiz de Montellano, P.R. Oxidation of α-meso-formylmesoheme by heme oxygenase. J. Biol. Chem. 272, 22008–22014 (1997).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. CCP4 Suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
de La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction method. Methods Enzymol. 276, 472–494 (1997).
Zhang, K.Y.J. & Main, P. The use of Sayre's equation with solvent flattening and histogram matching for phase extension and refinement of protein structures. Acta Crystallogr. A 46, 377–381 (1990).
Schuller, D.J. MAGICSQUASH: More versatile non-crystallographic averaging with multiple constraints. Acta Crystallogr. D 52, 425–434 (1996).
Navaza, J. AMORE—an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Jones, T.A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 115, 157–171 (1985).
Brünger, A.T. X-PLOR version 3.1: a system for X-ray crystallography and NMR (New Haven: Yale Univ. Press; 1992).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997).
Brünger, A.T. et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Evans, S.V. SETOR: hardware-lighted three dimensional solid model representation of macromolecules. J. Mol. Graphics 11, 134–138 (1993).
Sharp, K., Fine, R. & Honig, B. Computer simulations of the diffusion of a substrate to an active site of an enzyme. Science 236, 1460–1463 (1987).
Acknowledgements
Thanks to S. Tajbaksh for help with protein purification, to J.P. Cartailler for help with the GRASP figures, to B. Bhaskar, W.N. Lanzilotta, H. Li, C.S. Raman, I. Sevrioukova and M. Sundaramoorthy for helpful discussions, and to A. J. Greenwood for computational assistance. This work was supported by grants from the National Institutes of Health (T.L.P. and P.R.O.d.M.) and the National Science Foundation (T.L.P.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schuller, D., Wilks, A., Ortiz de Montellano, P. et al. Crystal structure of human heme oxygenase-1. Nat Struct Mol Biol 6, 860–867 (1999). https://doi.org/10.1038/12319
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/12319
This article is cited by
-
Non-canonical transcriptional regulation of heme oxygenase in Aedes aegypti
Scientific Reports (2019)
-
The Asp99–Arg188 salt bridge of the Pseudomonas aeruginosa HemO is critical in allowing conformational flexibility during catalysis
JBIC Journal of Biological Inorganic Chemistry (2018)
-
Comparative transcriptome analysis provides clues to molecular mechanisms underlying blue-green eggshell color in the Jinding duck (Anas platyrhynchos)
BMC Genomics (2017)
-
Function Coupling Mechanism of PhuS and HemO in Heme Degradation
Scientific Reports (2017)
-
Isocyanide or nitrosyl complexation to hemes with varying tethered axial base ligand donors: synthesis and characterization
JBIC Journal of Biological Inorganic Chemistry (2016)