Abstract
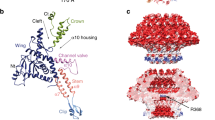
We have determined the three-dimensional structure of bacteriophage SPP1 portal protein (gp6) using electron microscopy at liquid-helium temperatures and angular reconstitution. The 13-fold symmetric gp6 oligomer is a turbine-shaped structure with three distinct regions: a conical stem with a central channel; the turbine wings region; and a fringe of small 'tentacles' at the end of the channel exposed to the viral head interior. The tentacle region appears flexible and may be associated with a particular function — sensing when the correct amount of DNA has been packaged. The three-dimensional structure of the gp6 SizA mutant, which packages a smaller chromosome, reveals significant differences in that region.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bazinet, C. & King, J. Annu. Rev. Microbiol. 39 , 109–129 (1985).
Valpuesta, J.M. & Carrascosa, J.L. Q. Rev. Biophys. 27, 107–155 ( 1994).
Tavares, P. et al. FEMS Microbiol. Rev. 17, 47– 56 (1995).
Dube, P., Tavares., P., Lurz, R. & van Heel, M. EMBO J. 12, 1303–1309 (1993).
Kocsis, E., Cerritelli, M.E., Trus, B.L., Cheng, N. & Steven, A.C. Ultramicroscopy 60, 219–228 (1995).
Tsuprun, V., Anderson, D. & Egelman, E.H. Biophys. J. 66, 2139– 2150 (1994).
van Heel, M., Orlova, E.V., Dube, P. & Tavares, P. EMBO J. 15, 4785–4788 (1996).
Jekow, P., Schaper, S., Günther, D., Tavares, P. & Hinrichs, W. Acta Crystallogr. D54 , 1008–1011 (1998).
Tavares, P. et al. J. Mol. Biol. 225, 81– 92 (1992).
van Heel, M. Ultramicroscopy 21, 111–124 (1987).
Zemlin, F., Beckmann, E. & van der Mast, K.D. Ultramicroscopy 63, 227– 238 (1996).
Black, L.W. Annu. Rev. Microbiol. 43, 267–292 (1989).
Turnquist, S., Simon, M., Egelman, E. & Anderson, D. Proc. Natl. Acad. Sci. USA 89, 10479–10483 (1992).
Fujisawa, H., Shibata, H. & Kato, H. Virology 185, 788– 794 (1991).
Krishna, T.S., Kong, X.P., Gary, S., Burgers, P.M. & Kuriyan, J. Cell 79, 1233– 1243 (1994).
Streisinger, G., Emrich, J. & Stahl, M.M. Proc. Nat. Acad. Sci. USA 57, 292 –295 (1967).
Tye, B.K., Huberman, J.A. & Botstein, D. J. Mol. Biol. 85, 501– 532 (1974).
Tavares, P., Lurz, R., Stiege, A., Rückert, B. & Trautner, T.A. J. Mol. Biol. 264, 954– 967 (1996).
Jekow, P, et al. J. Biochem. in the press ( 1999).
Dubochet, J. et al. Q. Rev. Biophys. 21, 129– 228 (1988).
Orlova, E.V. et al. J. Mol. Biol. 271, 417– 437 (1997).
van Heel, M., Harauz, G., Orlova, E.V., Schmidt, R. & Schatz, M. J. Struct. Biol. 116, 17– 24 (1996).
van Heel, M. Optik 82, 114–126 ( 1989).
Harauz, G. & van Heel, M. Optik 73, 146–156 (1986).
Radermacher, M. J. Elect. Microsc. Tech. 9, 359–394 (1988).
Müller, D.J., Engel, A., Carrascosa, J.L. & Vélez, M. EMBO J. 16, 2547–2553 ( 1997).
Acknowledgements
We are indebted to A. Isidro (ITQB, Oeiras) for communicating results prior to publication, and to R. Schmidt and M. Schatz for help with the IMAGIC image processing software. This work was supported in part by the Deutsche Forschungsgemeinschaft, and also by the EC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlova, E., Dube, P., Beckmann, E. et al. Structure of the 13-fold symmetric portal protein of bacteriophage SPP1 . Nat Struct Mol Biol 6, 842–846 (1999). https://doi.org/10.1038/12303
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/12303
This article is cited by
-
Three-dimensional structure of a viral genome-delivery portal vertex
Nature Structural & Molecular Biology (2011)
-
Structural framework for DNA translocation via the viral portal protein
The EMBO Journal (2007)
-
Structural changes of bacteriophage φ29 upon DNA packaging and release
The EMBO Journal (2006)
-
Molecular architecture of bacteriophage T4
Biochemistry (Moscow) (2004)
-
Molecular architecture of bacteriophage T4
Biochemistry (Moscow) (2004)