Abstract
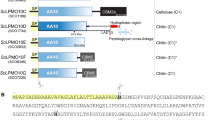
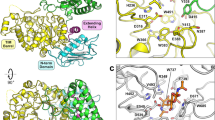
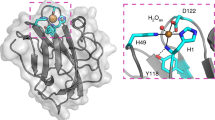
The recycling of photosynthetically fixed carbon in plant cell walls is a key microbial process. In anaerobes, the degradation is carried out by a high molecular weight multifunctional complex termed the cellulosome. This consists of a number of independent enzyme components, each of which contains a conserved dockerin domain, which functions to bind the enzyme to a cohesin domain within the protein scaffoldin protein. Here we describe the first three-dimensional structure of a fungal dockerin, the N-terminal dockerin of Cel45A from the anaerobic fungus Piromyces equi. The structure contains a novel fold of 42 residues. The ligand binding site consists of residues Trp 35, Tyr 8 and Asp 23, which are conserved in all fungal dockerins. The binding site is on the opposite side of the N- and C-termini of the molecule, implying that tandem dockerin domains, seen in the majority of anaerobic fungal plant cell wall degrading enzymes, could present multiple simultaneous binding sites and, therefore, permit tailoring of binding to catalytic demands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shoham, Y., Lamed, R. & Bayer, E.A. Trends Microbiol. 7, 275–281 (1999).
Bayer, E.A., Chanzy, H., Lamed, R. & Shoham, Y. Curr. Opin. Struct. Biol. 8, 548–557 (1998).
Yaron, S., Morag, E., Bayer, E.A., Lamed, R. & Shoham, Y. FEBS Lett. 360, 121–124 (1995).
Lytle, B.L., Myers, C., Kruus, K & Wu, J.H.D. J. Bacteriol. 178, 1200–1203 (1996).
Munn, E.A. In Anaerobic fungi: biology, ecology, and function. (eds Mountfort, D.O. & Orpin, C.G.) 47–105 (Marcel Dekker, New York; 1994).
Orpin, C.G. J. Gen. Microbiol. 123, 287–296 (1981).
Ali, B.R.S. et al. FEMS Microbiol. Lett. 125, 15–21 (1995).
Fanutti, C., Ponyi, T., Black, G.W., Hazlewood, G.P. & Gilbert, H.J. J. Biol. Chem. 270, 29314–29322 (1995).
Hazlewood, G.P. & Gilbert, H.J. In Carbohydrases from Trichoderma reesei and other microorganisms (eds Claeyssens, M., Nerinckx, W. & Piens, K.) 147–155 (Royal Society of Chemistry, Cambridge, UK; 1998).
Bayer, E.A., Shimon, L.J.W., Shoham, Y. & Lamed, R. J. Struct. Biol. 124, 221–234 (1998).
Li, X.-L., Chen, H. & Ljungdahl, L.G. Appl. Env. Microbiol. 63, 628–635 (1997).
Li, X.-L., Chen, H. & Ljungdahl, L.G. Appl. Environ. Microbiol. 63, 4721–4728 (1997).
Shimon, L.J.W. et al. Structure 5, 381–390 (1997).
Tavares, G.A., Souchon, H., Guérin, D.M., Lascombe, M.-B. & Alzari, P.M. In Carbohydrases from Trichoderma reesei and other microorganisms (eds Claeyssens, M., Nerinckx, W. & Piens, K.) 174–181 (Royal Society of Chemistry, Cambridge, UK; 1998).
Lytle, B.L., Volkman, B.F., Westler, W.M. & Wu, J.H.D. Arch. Biochem. Biophys. 379, 237–244 (2000).
Lytle, B.L., Volkman, B.F., Westler, W.M., Heckman, M.P. & Wu, J.H.D. J. Mol. Biol. 307, 745–753 (2001).
Eberhardt., R.Y., Gilbert, H.J. & Hazlewood, G.P. Microbiol. 146, 1999–2008 (2000).
Kataeva, I., Gugliemi, G. & Béguin, P. Biochem. J. 326, 617–624 (1997).
Tavares, G.A., Béguin, P. & Alzari, P.M. J. Mol. Biol. 273, 701–713 (1997).
Simpson, P.J., Xie, H., Bolam, D.N., Gilbert, H.J. & Williamson, M.P. J. Biol. Chem. 275, 41137–41142 (2000).
Mechaly, A. et al. J. Biol. Chem. 276, 9883–9888 (2001).
Kemp, P., Lander, D.J. & Orpin, C.G. J. Gen. Microbiol. 130, 27–37 (1984).
Nilges, M. J. Mol. Biol. 245, 645–660 (1995).
Baxter, N.J. & Williamson, M.P. J. Biomol. NMR 9, 359–369 (1997).
Simpson, P.J, et al. Structure 7, 853–864 (1999).
Sorimachi, K., Le Gal-Coëffet, M-F., Williamson, G., Archer, D.B. & Williamson, M.P. Structure 5, 647–661 (1997).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Acknowledgements
We thank the Biotechnology and Biological Sciences Research Council (BBSRC) for project grants and CASE studentships, Finnfeeds International for financial support, and BBSRC and the Wellcome Trust for equipment grants. The Krebs Institute is a BBSRC Centre. M.P.W., S.R. and P.J.S .are members of the BBSRC-funded North of England Structural Biology Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raghothama, S., Eberhardt, R., Simpson, P. et al. Characterization of a cellulosome dockerin domain from the anaerobic fungus Piromyces equi. Nat Struct Mol Biol 8, 775–778 (2001). https://doi.org/10.1038/nsb0901-775
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0901-775
This article is cited by
-
Structure and enzymatic characterization of CelD endoglucanase from the anaerobic fungus Piromyces finnis
Applied Microbiology and Biotechnology (2023)
-
Two-site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b
Nature Chemical Biology (2020)
-
Enhanced features of Dictyoglomus turgidum Cellulase A engineered with carbohydrate binding module 11 from Clostridium thermocellum
Scientific Reports (2018)
-
A parts list for fungal cellulosomes revealed by comparative genomics
Nature Microbiology (2017)
-
Transcriptomic analysis of lignocellulosic biomass degradation by the anaerobic fungal isolate Orpinomyces sp. strain C1A
Biotechnology for Biofuels (2015)