Abstract
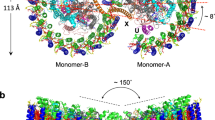
Escherichia coli osmosensor EnvZ is a protein histidine kinase that plays a central role in osmoregulation, a cellular adaptation process involving the His-Asp phosphorelay signal transduction system. Dimerization of the transmembrane protein is essential for its autophosphorylation and phosphorelay signal transduction functions. Here we present the NMR-derived structure of the homodimeric core domain (residues 223–289) of EnvZ that includes His 243, the site of autophosphorylation and phosphate transfer reactions. The structure comprises a four-helix bundle formed by two identical helix-turn-helix subunits, revealing the molecular assembly of two active sites within the dimeric kinase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Egger, L.A., Park, H. & Inouye, M. Genes Cells 2, 167–184 (1997).
Mizuno, T. J. Biochem. (Tokyo) 123, 555–563 (1998).
Swanson, R.V., Alex, L.A. & Simon, M.I. Trends Biochem. Sci. 19, 485–490 (1994).
Forst, S., Comeau, D., Norioka, S. & Inouye, M. J. Biol. Chem. 262, 16433–16438 (1987).
Forst, S.A. & Roberts, D.L. Res. Microbiol. 145, 363–373 (1994).
Park, H., Saha, S.K. & Inouye, M. Proc. Natl. Acad. Sci. USA 95, 6728–6732 (1998).
Hidaka, Y., Park, H. & Inouye, M. FEBS Lett. 400, 238–242 (1997).
Kay, L.E. Prog. Biophys. Mol. Biol. 63, 277–299 (1995).
Zhou, H., Lowry, D.F., Swanson, R.V., Simon, M.I. & Dahlquist, F.W. Biochemistry 34, 13858–13870 (1995).
Kato, M., Mizuno, T., Shimizu, T. & Hakoshima, T. Cell 88, 717–723 (1997).
Varughese, K.I., Madhusudan, Zhou, X.Z., Whiteley, J.M. & Hoch, J.A. Mol. Cell 2, 485–493 (1998).
Bilwes, A.M., Alex, L.A., Crane, B.R. & Simon, M.I. Cell 96, 131–141 (1999).
Tanaka, T. et al. Nature 396, 88–92 (1998).
Hsing, W. & Silhavy, T.J. J. Bacteriol. 179, 3729–3735 (1997).
Hsing, W., Russo, F.D., Bernd, K.K. & Silhavy, T.J. J. Bacteriol. 180, 4538–4546 (1998).
Russo, F.D. & Silhavy, T.J. J. Mol. Biol. 222, 567–580 (1991).
Delgado, J., Forst, S., Harlocker, S. & Inouye, M. Mol. Microbiol. 10, 1037–1047 (1993).
Spera, S., Ikura, M. & Bax, A. J. Biomol. NMR 1, 155–165 (1991).
Bax, A. et al. Methods Enzymol 239, 79–105 (1994).
Delaglio, F. et al. J. Biomol. NMR 6, 277–293 (1995).
Garrett, D.S., Powers, R., Gronenborn, A. & Clore, G.M. J. Magn. Reson. 95, 214–220 (1991).
Wüthrich, K., Billeter, M. & Braun, W. J. Mol. Biol. 169, 949–961 (1983).
Venters, R.A., Farmer II, B.T., Fierke, C.A. & Spicer, L.D. J. Mol. Biol. 264, 1101–1116 (1996).
Nilges, M., Clore, G.M. & Gronenborn, A.M. FEBS Lett. 229, 317–324 (1988).
Nilges, M. J. Mol. Biol. 245, 645–660 (1995).
Brünger, A.T. X-PLOR Version3.1: A system for X-ray crystallography and NMR (Yale University Press, New Haven, Connecticut; 1993).
Koradi, R., Billeter, M. & Wüthrich, K. J. Mol. Graph. 14, 51–55, 29–32 (1996).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
Zhang, Z. et al. Nucleic. Acids Res. 26, 3986–3990 (1998).
Acknowledgements
We thank L. Kay for providing NMR pulse sequences, D. Garrett for helpful instructions on PIPP/STAPP, M. Osawa for providing software used in structure calculation, K. Yap for calculation of interhelical angles, and S. Bagby for critical reading of the manuscript. This work was supported by grants to T.T. from JSPS, to M. Inouye from the NIH, and to M. Ikura from the Howard Hughes Medical Institute. R.I. and D.L. acknowledge HFSP postdoctoral fellowships, and C.T. a postgraduate fellowship from the Ministry of Education, Science and Culture of Japan and from the TARA, University of Tsukuba. M. Ikura is an HHMI International esearch Scholar and a Medical Research Council of Canada Scientist.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tomomori, C., Tanaka, T., Dutta, R. et al. Solution structure of the homodimeric core domain of Escherichia coli histidine kinase EnvZ . Nat Struct Mol Biol 6, 729–734 (1999). https://doi.org/10.1038/11495
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11495
This article is cited by
-
Artificial signaling in mammalian cells enabled by prokaryotic two-component system
Nature Chemical Biology (2020)
-
Essential Two-Component Systems Regulating Cell Envelope Functions: Opportunities for Novel Antibiotic Therapies
The Journal of Membrane Biology (2018)
-
Mechanism of early light signaling by the carboxy-terminal output module of Arabidopsis phytochrome B
Nature Communications (2017)
-
Angucycline antibiotic waldiomycin recognizes common structural motif conserved in bacterial histidine kinases
The Journal of Antibiotics (2017)
-
Molecular Characterization of Ethylene Response Sensor 1 (BoERS1) in Bambusa oldhamii
Plant Molecular Biology Reporter (2016)