Abstract
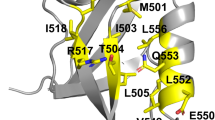
The importance of chain connectivity in determining protein function and stability can be examined by breaking the peptide backbone using a technique such as circular permutation. Cleavage at certain positions results in a complete loss of the ability of the protein to fold. When such cleavage sites occur sequentially in the primary structure, we call the region a ‘folding element’, a new concept that could assist in our understanding of the protein folding problem. The folding elements of dihydrofolate reductase have been assigned by conducting a systematic circular permutation analysis in which the peptide backbone was sequentially broken between every pair of residues in the protein. The positions of folding elements do not appear to correspond to secondary structure motifs, substrate or coenzyme binding sites, or accessible surface area. However, almost all of the amino acid residues known to be involved in early folding events are located within the folding elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cordes, M.H.J., Davidson, A.R. & Sauer, R.T. Sequence space, folding and protein design. Curr. Opin. Struct. Biol. 6, 3–10 (1996).
Matsuura, T., et al. Evolutionary molecular engineering by random elongation mutagenesis. Nature Biotechnol. 17, 58–61 (1999).
Luger, K., Hommel, U., Herold, M., Hofsteenge, J. & Kirschner, K. Correct folding of circularly permuted variants of a beta alpha barrel enzyme in vivo. Science 243, 206–210 (1989).
Viguera, A.R., Blanco, F.J. & Serrano, L. The order of secondary structure elements does not determine the structure of a protein but does affect its folding kinetics. J. Mol. Biol. 247, 670–681 (1995).
Taniuchi, H. & Anfinsen, C.B. Simultaneous formation of two alternative enzymology active structures by complementation of two overlapping fragments of staphylococcal nuclease. J. Biol. Chem. 246, 2291–2301 (1971).
de Prat-Gay, G. Association of complementary fragments and the elucidation of protein folding pathways. Protein Eng. 9, 843–847 (1996).
Chaffotte, A.F., Li, J.H., Georgescu, R.E., Goldberg, M.E. & Tasayco, M.L. Recognition between disordered states: kinetics of the self-assembly of thioredoxin fragments. Biochemistry 36, 16040–16048 (1997).
Gegg, C.V., Bowers, K.E. & Matthews, C.R. Probing minimal independent folding units in dihydrofolate reductase by molecular dissection. Protein Sci. 6, 1885–1892 (1997).
Itzhaki, L.S., Otzen, D.E. & Fersht, A.R. The structure of the transition state for folding of chymotrypsin inhibitor 2 analysed by protein engineering methods: evidence for a nucleation-condensation mechanism for protein folding. J. Mol. Biol. 254, 260–288 (1995).
Nakamura, T. & Iwakura, M. Circular permutation analysis as a method for distinction of functional elements in the M20 loop of Escherichia coli dihydrofolate reductase. J. Biol. Chem. 274, 19041–19047 (1999).
Graf, R. & Schachman, H.K. Random circular permutation of genes and expressed polypeptide chains: application of the method to the catalytic chains of aspartate transcarbamoylase. Proc. Natl. Acad. Sci. USA 93, 11591–11596 (1996)
Hennecke, J., Sebbel, P. & Glockshuber, R. Random circular permutation of DsbA reveals segments that are essential for protein folding and stability. J. Mol. Biol. 286, 1197–1215 (1999).
Blakley, R.L. Dihydrofolate reductase. In Folates and pteridines Vol. 1 (eds, Blakley, R.L. & Benkovic, S.J.), 191–253 (Wiley, New York; 1984).
Uwajima, T., et al. Chemo-enzymatic synthesis of optically pure l-leucovorin, an augmentor of 5-fluorouracil cytotoxicity against cancer. Biochem. Biophys. Res. Commun. 171, 684–689 (1990).
Bystroff, C. & Kraut, J. Crystal structure of unliganded Escherichia coli dihydrofolate reductase. Ligand-induced conformational changes and cooperativity in binding. Biochemistry 30, 2227–2239 (1991).
Iwakura, M. & Nakamura, T. Effects of the length of a glycine linker connecting the N- and C-termini of a circularly permuted dihydrofolate reductase. Protein Eng. 11, 707–713 (1998).
Iwakura, M., Shimura, Y. & Tsuda, K. Construction of plasmid vectors for cloning of promoters using the dihydrofolate reductase gene of Escherichia coli K-12. J. Biochem. 93, 927–930 (1983).
Iwakura, M., Jones, B.E., Falzone, C.J. & Matthews, C.R. Collapse of parallel folding channels in dihydrofolate reductase from Escherichia coli by site-directed mutagenesis. Biochemistry 32, 13566–13574 (1993).
Jennings, P.A., Finn, B.E., Jones, B.E. & Matthews, C.R. A reexamination of the folding mechanism of dihydrofolate reductase from Escherichia coli : verification and refinement of a four-channel model. Biochemistry 32, 3783–3789 (1993).
Kuwajima, K., Garvey, E.P., Finn, B.E., Matthews, C.R. & Sugai, S. Transient intermediates in the folding of dihydrofolate reductase as detected by far-ultraviolet circular dichroism spectroscopy. Biochemistry 30, 7693–7703 (1991).
Jones, B.E. & Matthews, C.R. Early intermediates in the folding of dihydrofolate reductase from Escherichia coli detected by hydrogen exchange and NMR. Protein Sci. 4, 167–177 (1995).
Anfinsen, C.B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Iwakura, M., et al. Dihydrofolate reductase as a new ‘affinity handle’. J. Biochem. 111, 37–45 (1992).
Touchette, N.A., Perry, K.P. & Matthews, C.R. Folding of dihydrofolate reductase from Escherichia coli. Biochemistry 25, 5445–5452 (1986).
Hillcoat, B.L., Nixon, P.F. & Blakely, R.L. Effect of substrate decomposition on the spectrophotometric assay of dihydrofolate reductase. Anal. Biochem. 21, 178–189 (1967).
Morrison, J.F. & Stone, S.R. Mechanism of the reaction catalyzed by dihydrofolate reductase from Escherichia coli: pH and deuterium isotope effects with NADPH as the variable substrate. Biochemistry 27, 5499–5506 (1988).
Iwakura, M., Takenawa, T. & Nakamura, T. Topological mutation of Escherichia coli dihydrofolate reductase. J. Biochem. 124, 769–777 (1998).
Sawaya, M.R. & Kraut, J.B. Loop and subdomain movements in the mechanism of Escherichia coli dihydrofolate reductase: crystallographic evidence. Biochemistry 36, 586–603 (1997).
Merritt, E.A. & Bacon, D.J. MolScript: the official web site. http://www.avatar.se/molscript/
Acknowledgements
We thank N. Furuya, A. Fujisawa, J. Suzuki and T. Takenawa, National Institute of Bioscience and Human Technology, for protein purification, DNA sequencing, and mass spectrometry measurements. We are grateful to C.R. Matthews and V.F. Smith, the Pennsylvania State University, for continuous discussion and encouragement and critical review of this article. This research was supported in part by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwakura, M., Nakamura, T., Yamane, C. et al. Systematic circular permutation of an entire protein reveals essential folding elements. Nat Struct Mol Biol 7, 580–585 (2000). https://doi.org/10.1038/76811
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76811
This article is cited by
-
CirPred, the first structure modeling and linker design system for circularly permuted proteins
BMC Bioinformatics (2021)
-
Protein rethreading: A novel approach to protein design
Scientific Reports (2016)
-
Conserved structural elements in the V3 crown of HIV-1 gp120
Nature Structural & Molecular Biology (2010)
-
Evolution of new protein topologies through multistep gene rearrangements
Nature Genetics (2006)
-
A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-κB
Nature Structural & Molecular Biology (2005)