Abstract
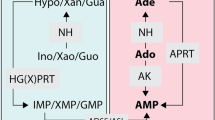
The proposed transition state for hypoxanthine-guanine phosphoribosyltransferases (HGPRTs) has been used to design and synthesize powerful inhibitors that contain features of the transition state. The iminoribitols (1S)-1-(9-deazahypoxanthin-9-yl)-1,4-dideoxy-1, 4-imino-D-ribitol 5-phosphate (immucillinHP) and (1S)-1-(9-deazaguanin-9-yl)-1,4-dideoxy-1, 4-imino-D-ribitol 5-phosphate (immucillinGP) are the most powerful inhibitors yet reported for both human and malarial HGPRTs. Equilibrium binding constants are >1,000-fold tighter than the binding of the nucleotide substrate. The NMR spectrum of malaria HGXPRT in the Michaelis complex reveals downfield hydrogen-bonded protons. The chemical shifts move farther downfield with bound inhibitor. The inhibitors are lead compounds for species-specific antibiotics against parasitic protozoa. The high-resolution crystal structure of human HGPRT with immucillinGP is reported in the companion paper.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Berens, R.L., Krug, E.C. & Marr, J.J. in Biochemistry of parasitic organisms and its molecular foundations (eds Marr, J.J. & Muller, M.) 89– 117 (Academic Press, London; 1995).
Berman, P.A., Human, L. & Freese, J.A. Xanthine oxidase inhibits growth of Plasmodium falciparum in human erythrocytes in vitro. J. Clin. Invest. 88, 1848–1855 (1991).
Nchinda, T.C. Malaria: a reemerging disease in Africa. Emerg. Infect. Dis. 4, 398–403 (1998).
Jadhav, A.L., Townsend, L.B. & Nelson, J.A. Inhibition of hypoxanthine-guanine phosphoribosyltransferase. Biochem. Pharmacol. 28, 1057– 1062 (1979).
Bennett, L.L. et al. Inhibition of utilization of hypoxanthine and guanine in cells treated with the carbocyclic analog of adenosine. Phosphates of carbocyclic nucleoside analogs as inhibitors of hypoxanthine (guanine) phosphoribosyltransferase. Mol. Pharmacol. 27, 666– 675 (1985).
Eakin, A.E., Guerra, A., Focia, P.J., Torres-Martinez, J. & Craig, S.P. Hypoxanthine phosphoribosyltransferase from Trypanosoma cruzi as a target for structure-based inhibitor design: crystallization and inhibition studies with purine analogs. Antimicrob. Agents Chemother. 41, 1686–1692 (1997).
Somoza, J.R. et al. Rational design of novel antimicrobials blocking purine salvage in a parasitic protozoan. Biochemistry 37, 5344–5348 (1998).
Queen, S.A., VanderJagt, D.L. & Reyes, P. In vitro susceptibilities of Plasmodium falciparum to compounds which inhibit nucleotide metabolism. Antimicrob. Agents Chemother. 34, 1393–1398 (1990).
Tao, W., Grubmeyer, C. & Blanchard, J.S. Transition state structure of Salmonella typhimurium orotate phosphoribosyltransferase. Biochemistry 35, 14–21 (1996).
Schramm, V.L. Enzymatic N-riboside scission in RNA and RNA precursors. Curr. Opin. Chem. Biol. 1, 323–331 (1997).
Miles, R.W., Tyler, P.C., Furneaux, R.H., Bagdassarian, C.K. & Schramm, V.L. One-third-the-sites transition state inhibitors for purine nucleoside phosphorylase. Biochemistry 37, 8615–8621 (1998).
Focia, P.J., Craig, S.P. & Eakin, A.E. Approaching the transition state in the crystal structure of a phosphoribosyltransferase. Biochemistry 37, 17120–17127 (1998).
Shi, W. et al. The 2.0 Å structure of human hypoxanthine-guanine phosphoribosyltransferase in complex with a transition-state analog inhibitor. Nature Struct. Biol. 6, ´–´ (1999).
Xu, Y. & Grubmeyer, C. Catalysis in human hypoxanthine-guanine phosphoribosyltransferase: Asp-137 acts as a general acid/base. Biochemistry 37, 4114–4124 (1998).
Xu, Y., Eads, J.C., Sacchettini, J.C. & Grubmeyer, C. Kinetic mechanism of human hypoxanthine-guanine phosphoribosyltransferase: rapid phosphoribosyl transfer chemistry. Biochemistry 36, 3700–3712 (1997).
Morrison, J.F. & Walsh, C.T. The behavior and significance of slow-binding enzyme inhibitors. Adv. Enzymol. 61, 201–301 (1988).
Cleland, W.W. & Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 264, 1887– 1890 (1994).
Shan, S-O. & Herschlag, D. The change in hydrogen bond strength accompanying charge rearrangement: implications for enzymatic catalysis. Proc. Natl. Acad. Sci. USA 93, 14474–14479 (1996).
Gerlt, J.A. & Gassman, P.G. An explanation for rapid enzyme-catalyzed proton abstraction from carbon acids: importance of late transition states in concerted mechanisms. J. Am. Chem. Soc. 115, 11552–11568 (1993).
Halkides, C.J., Wu, Y.Q. & Murry, C.J. A low-barrier hydrogen bond in subtilisin: 2H and 15N NMR studies with peptidyl trifluoromethyl ketones. Biochemistry 35, 15941– 15948 (1996).
Kahyaoglu, K.A. et al. Low barrier hydrogen bond is absent in the ground state but is present in a transition-state complex in the prolyl oligopeptidase family of serine proteases. J. Biol. Chem. 272, 25547–25554 (1997).
Zhao, Q., Abeygunawardana, C., Gittis, A.G. & Mildvan, A.S. Hydrogen bonding at the active site of delta 5,3-ketosteroid isomerase. Biochemistry 36, 14616–14626 (1997).
Eigen, M. Proton transfer, acid-base catalysis, and enzymatic hydrolysis. Part I: Elementary processes. Angew. Chem. Int. Edn. Engl. 33, 1–72 (1964).
Horenstein, B.A., Zabinski, R.F. & Schramm, V.L. A new class of C-nucleoside analogs. 1-(S)-aryl-1,4-dideoxy-1,4-imino-D-ribitols, transition state analog inhibitors of nucleoside hydrolase. Tetrahedr. Lett. 34, 7213–7216 (1993).
Furneaux, R.H., Limberg, G., Tyler, P.C. & Schramm, V.L. Synthesis of transition state inhibitors for N-riboside hydrolases and transferases. Tetrahedron 53, 2915–2930 (1997).
Lim, M-I., Ren, W-Y, Otter, B.A. & Kline, R.S. Synthesis of 9-deazaguanosine and other new pyrrolo[3,2-d]pyrimidine C-nucleosides. J. Org. Chem. 48, 780– 788 (1983).
Bennett, L.W., Comber, R.N. & Secrist, J.A. Differences in the metabolism and metabolic effects of the carbocyclic adenosine analogs, neplanocin A and aristeromycin. Mol. Pharmacol. 29, 383–390 (1986).
Merkler, D.J. & Schramm, V.L. A preparative method for the enzymatic 5'-monophosphorylation of nucleosides. Anal. Biochem. 167, 148–153 (1987).
Brennard, J., Konecki, D.S. & Caskey, C.T. Expression of human and Chinese hamster hypoxanthine-guanine phosphoribosyltransferase cDNA recombinants in cultured Lesch–Nyhan and Chinese hamster fibroblasts. J. Biol. Chem. 258, 9593–9598 (1983).
Eads, J.C., Scapin, G., Xu, Y., Grubmeyer, C. & Sacchettini, J.C. The crystal structure of human hypoxanthine-guanine phosphoribosyltransferase with bound GMP. Cell 78, 325–334 (1994).
Schramm, V.L. Comparison of initial velocity and binding data for allosteric adenosine monophosphate nucleosidase. J. Biol. Chem. 251, 3417– 3424 (1976).
Plateau, P. & Guéron, M. Exchangeable proton NMR without base-line distortion, using new strong-pulse sequences. J. Am. Chem. Soc. 104, 7310–7311 (1982).
Bax, A., Griffey, R.H. & Hawkins, B.L. Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J. Magn. Reson. 55, 301–315 (1983).
Horenstein, B.A. & Schramm, V.L. Correlation of the molecular electrostatic potential surface of an enzymatic transition state with novel transition state inhibitors. Biochemistry 32, 9917–9925 (1993).
Acknowledgements
Research grants and training grants from the National Institutes of Health, and the New Zealand Foundation for Research, Science and Technology supported this research. The NMR facility was supported in part by grants from the NSF and the Howard Hughes Medical Institute Biomedical Research Support Program for Medical Schools. We thank A. Sauve for the enzymatic phosphorylation of [7-15N]immucillinHP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C., Tyler, P., Furneaux, R. et al. Transition-state analogs as inhibitors of human and malarial hypoxanthine-guanine phosphoribosyltransferases. Nat Struct Mol Biol 6, 582–587 (1999). https://doi.org/10.1038/9367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9367
This article is cited by
-
Antimalarial drug targets in Plasmodium falciparum predicted by stage-specific metabolic network analysis
BMC Systems Biology (2010)
-
Heterologous expression of plasmodial proteins for structural studies and functional annotation
Malaria Journal (2008)