Abstract
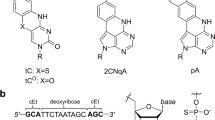
2'-O-(2-Methoxyethyl)-RNA (MOE-RNA) is a nucleic acid analog with promising features for antisense applications. Compared with phosphorothioate DNA (PS-DNA), the MOE modification offers improved nuclease resistance, enhanced RNA affinity, improved cellular uptake and intestinal absorption, reduced toxicity and immune stimulation. The crystal structure of a fully modified MOE-RNA dodecamer duplex (CGCGAAUUCGCG) was determined at 1.7 Å resolution. In the majority of the MOE substituents, the torsion angle around the ethylene alkyl chain assumes a gauche conformation. The conformational preorganization of the MOE groups is consistent with the improved RNA affinity and the extensive hydration of the substituents could play a role in the improved cellular uptake of MOE-RNA. A specific hydration pattern that bridges substituent and phosphate oxygen atoms in the minor groove of MOE-RNA may explain its high nuclease resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Mesmaeker, A., Häner, R., Martin, P. & Moser, H. E. Acc. Chem. Res. 28, 366–374 (1995).
Crooke, S.T. In Handbook of experimental pharmacology, vol. 131, Antisense Research and Applications (ed. Crooke, S.T.) 1–50 (Springer-Verlag, Berlin and Heidelberg, 1998).
Cook, P. D. Annu. Med. Rep. Chem. 33, 313–325 (1998).
Altmann, K.-H. et al. Biochem. Soc. Trans. 24, 630–637 (1996).
Altmann, K.-H. et al. Chimia 50, 168–176 (1996).
Martin, P. Helv. Chim. Acta 78, 486–504 (1995).
Freier, S. M. & Altmann, K.-H. Nucleic Acids Res. 25, 4429–4443 (1997).
Akhtar, S. & Agrawal, S. Trends Pharmacol. Sci. 18, 12–18 (1997).
Greig, M. J., Gaus, H., Cummins, L. L., Sasmor, H. & Griffey, R.H. J. Am. Chem. Soc. 117, 10765–10766 (1995).
Baker, B. F. et al. J. Biol. Chem. 272, 11994–12000 (1997).
Agrawal, S. Trends Biotechnol. 14, 376–387 (1996).
Gao, W. Y., Storm, C., Egan, W. & Cheng, Y. C. Mol. Pharmacol. 43, 45–50 (1993).
Wallace, T. L., Bazemore, S. A., Kornbrust, D. J. & Cossum, P. A. J. Pharmacol. Exp. Ther. 278, 1306–1312 (1996).
Agrawal, S. et al. Antisense Nucleic Acid Drug Dev. 7, 575–584 (1997).
Henry, S. P. et al. Anti-Cancer Drug Design 12, 1–14 (1997).
Henry, S. P., Monteith, D., Bennett, F. & Levin, A. A. Anti-Cancer Drug Design 12, 409–420 (1997).
Levin, A. A. et al. In Handbook of experimental pharmacology, vol. 131, Antisense research and applications (ed. Crooke, S.T.) 169–215 (Springer Verlag, Berlin and Heidelberg; 1998).
Saenger, W. Principles of nucleic acid structure (Springer Verlag, New York; 1984).
Quigley, G. J., Teeter, M. M. & Rich, A. Proc. Natl. Acad. Sci. U.S.A. 75, 64–68 (1978).
Cate, J. H. & Doudna, J. A. Structure 4, 1221–1229 (1996).
Scott, W. G., Finch, J. T. & Klug, A. Cell 81, 991–1002 (1995).
Portmann, S., Usman, N. & Egli, M. Biochemistry 34, 7569–7575 (1995).
Correll, C. C., Freeborn, B., Moore, P. B. & Steitz, T. A. Cell 91, 705–712 (1997).
Deslongchamps, P. Stereoelectronic effects in organic chemistry (Tetrahedron Organic Chemistry Series, Pergamon, Oxford; 1983).
Cummins, L. L. et al. Nucleic Acids Res. 23, 2019–2024 (1995).
Tereshko, V. et al. Biochemistry 37, 10626–10634 (1998).
Lind, K. E., Mohan, V., Manoharan, M. & Ferguson, D. M. Nucleic Acids Res. 26, 3694–3699 (1998).
Egli, M., Portmann, S. & Usman, N. Biochemistry 35, 8489–8494 (1996).
Schneider, B., Patel, K. & Berman, H. M. Biophys. J. 75, 2422–2434 (1998).
Khatsenko, O., Morgan, R. & Geary, R. Pharm. Res. 16 in the press (1999).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Brünger, A. T. X-PLOR 3.1, A system for X-ray crystallography and NMR (Yale University Press, New Haven, Conneticut; 1993).
Parkinson, G., Vojtechovsky, J., Clowney, L., Brünger, A. T. & Berman, H. M. Acta Crystallogr. D 52, 57–64 (1996).
Marky, L. A. & Breslauer, K. J. Biopolymers 26, 1601–1620 (1987).
Lavery, R. & Sklenar, H. J. J. Biomol. Struct. Dyn. 6, 655–667 (1989).
Brünger, A. T. Nature 355, 472–475 (1992).
Acknowledgements
This work was supported through a grant by the National Institutes of Health to M.E. We are indebted to Northwestern University Medical School for supporting structural research through the Macromolecular Crystallography and Biochemical Computation Resource and to the Northwestern Drug Discovery Program. We thank B. Ross and his group at Isis Pharmaceuticals Inc. for providing the phosphoramidites for the dodecamer synthesis and S.T. Wallace for technical assistance. Part of the data collections were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) Synchrotron Research Center located at Sector 5 of the Advanced Photon Source at Argonne National Laboratory, Argonne, Illinois. DND-CAT is supported by the E.I. DuPont de Nemours & Co., The Dow Chemical Company, the U.S. National Science Foundation and the State of Illinois.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Teplova, M., Minasov, G., Tereshko, V. et al. Crystal structure and improved antisense properties of 2'-O-(2-methoxyethyl)-RNA. Nat Struct Mol Biol 6, 535–539 (1999). https://doi.org/10.1038/9304
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9304
This article is cited by
-
A two-residue nascent-strand steric gate controls synthesis of 2′-O-methyl- and 2′-O-(2-methoxyethyl)-RNA
Nature Chemistry (2023)
-
Artificial genetic polymers against human pathologies
Biology Direct (2022)
-
Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in amyotrophic lateral sclerosis
Nature Medicine (2022)
-
Intrastrand backbone-nucleobase interactions stabilize unwound right-handed helical structures of heteroduplexes of L-aTNA/RNA and SNA/RNA
Communications Chemistry (2020)
-
DNA/RNA heteroduplex oligonucleotide for highly efficient gene silencing
Nature Communications (2015)