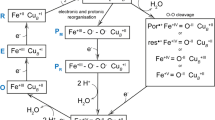
Abstract
Two models have been proposed to describe the folding pathways of proteins. The framework model assumes the initial formation of the secondary structures whereas the hydrophobic collapse model supposes their formation after the collapse of backbone structures. To differentiate between these models for real proteins, we have developed a novel CD spectrometer that enables us to observe the submillisecond time frame of protein folding and have characterized the timing of secondary structure formation in the folding process of cytochrome c (cyt c). We found that ∼20% of the native helical content was organized in the first phase of folding, which is completed within milliseconds. Furthermore, we suggest the presence of a second intermediate, which has α-helical content resembling that of the molten globule state. Our results indicate that many of the α-helices are organized after collapse in the folding mechanism of cyt c.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Anfinsen, C.B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Levinthal, C. Are there pathways for protein folding? J. Chim. Phys. 65, 44–45 (1968).
Dill, K.A. & Chan, H.S. From Levinthal to pathways to funnels. Nature Struct. Biol. 4, 10–19 (1997).
Baldwin, R.L. & Rose, G.D. Is protein folding hierarchic? I. Local structure and peptide folding. Trends Biochem. Sci. 24, 26–33 (1999).
Baldwin, R.L. & Rose, G.D. Is protein folding hierarchic? II. Folding intermediates and transition states. Trends Biochem. Sci. 24, 77–83 (1999).
Kim, P.S. & Baldwin, R.L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu. Rev. Biochem. 51, 459–489 (1982).
Dill, K.A. Theory for the folding and stability of globular proteins. Biochemistry 24, 1501–1509 (1985).
Sivaraman, T. et al. Secondary structure formation is the earliest structural event in the refolding of an all β-sheet protein. Biochem. Biophys. Res. Commun. 260, 284–288 (1999).
Agashe, V.R., Shastry, M.C. & Udgaonkar, J.B. Initial hydrophobic collapse in the folding of barstar. Nature 377, 754–757 (1995).
Sosnick, T.R., Mayne, L., Hiller, R. & Englander, S.W. The barriers in protein folding. Nature Struct. Biol. 1, 149–156 (1994).
Jennings, P.A. & Wright, P.E. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 262, 892–896 (1993).
Chaffotte, A.F., Guillou, Y. & Goldberg, M.E. Kinetic resolution of peptide bond and side chain far-UV circular dichroism during the folding of hen egg white lysozyme. Biochemistry 31, 9694–9702 (1992).
Ptitsyn, O.B. Molten globule and protein folding. Adv. Protein Chem. 47, 83–229 (1995).
Kuwajima, K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins 6, 87–103 (1989).
Shastry, M.C., Luck, S.D. & Roder, H. A continuous-flow capillary mixing method to monitor reactions on the microsecond time scale. Biophys. J. 74, 2714–2721 (1998).
Takahashi, S. et al. Folding of cytochrome c initiated by submillisecond mixing. Nature Struct. Biol. 4, 44–50 (1997).
Chan, C.K. et al. Submillisecond protein folding kinetics studied by ultrarapid mixing. Proc. Natl. Acad. Sci. USA 94, 1779–1784 (1997).
Bushnell, G.W., Louie, G.V. & Brayer, G.D. High-resolution three-dimensional structure of horse heart cytochrome c. J. Mol. Biol. 214, 585–595 (1990).
Shastry, M.C.R. & Roder, H. Evidence for barrier-limited protein folding kinetics on the microsecond time scale. Nature Struct. Biol. 5, 385–392 (1998).
Pollack, L. et al. Compactness of the denatured state of a fast-folding protein measured by submillisecond small-angle X-ray scattering. Proc. Natl. Acad. Sci. USA 96, 10115–10117 (1999).
Yeh, S.R., Takahashi, S., Fan, B. & Rousseau, D.L. Ligand exchange during cytochrome c folding. Nature Struct. Biol. 4, 51–56 (1997).
Sauder, J.M. & Roder, H. Amide protection in an early folding intermediate of cytochrome c. Folding Design 3, 293–301 (1998).
Elöve, G.A., Chaffotte, A.F., Roder, H. & Goldberg, M.E. Early steps in cytochrome c folding probed by time-resolved circular dichroism and fluorescence spectroscopy. Biochemistry 31, 6876–6883 (1992).
Chen, E., Wittung-Stafshede, P. & Kliger, D.S. Far-UV time-resolved circular dichroism detection of electron-transfer-triggered cytochrome c folding. J. Am. Chem. Soc. 121, 3811–3817 (1999).
Takahashi, S., Akiyama, S., Ishimori, K. & Morishima, I. CD measurements on the early folding intermediate of cytochrome c using the fast flow mixer. In Old and new views of protein folding (eds Kuwajima, K. & Arai, M.) 75–84 (Elsevier Science, Amsterdam; 1999).
Greenfield, N. & Fasman, G.D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8, 4108–4116 (1969).
Yeh, S.R., Han, S. & Rousseau, D.L. Cytochrome c folding and unfolding: a biphasic mechanism. Acc. Chem. Res. 31, 727–736 (1998).
Goldbeck, R.A. & Kliger, D.S. Nanosecond time-resolved absorption and polarization dichroism spectroscopies. Methods Enzymol. 226, 147–177 (1993).
Shastry, M.C.R., Sauder, J.M. & Roder, H. Kinetic and structural analysis of submillisecond folding events in cytochrome c. Acc. Chem. Res. 31, 717–725 (1998).
Sosnick, T.R., Shtilerman, M.D., Mayne, L. & Englander, S.W. Ultrafast signals in protein folding and the polypeptide contracted state. Proc. Natl. Acad. Sci. USA 94, 8545–8550 (1997).
Mok, Y.K., Kay, C.M., Kay, L.E. & Forman-Kay, J. NOE data demonstrating a compact unfolded state for an SH3 domain under non-denaturing conditions. J. Mol. Biol. 289, 619–638 (1999).
Lecomte, J.T. & Falzone, C.J. Where U and I meet. Nature Struct. Biol. 6, 605–608 (1999).
Yeh, S.R. & Rousseau, D.L. Folding intermediates in cytochrome c. Nature Struct. Biol. 5, 222–228 (1998).
Colón, W., Wakem, L.P., Sherman, F. & Roder, H. Identification of the predominant non-native histidine ligand in unfolded cytochrome c. Biochemistry 36, 12535–12541 (1997).
Fersht, A. Kinetics of protein folding. In Structure and mechanism in protein science (Julet, M.R. and Hadler G.L., eds) 540–572 (W.H. Freeman and Company, New York; 1998).
Roder, H., Elöve, G.A. & Englander, S.W. Structural characterization of folding intermediates in cytochrome c by H-exchange labelling and proton NMR. Nature 335, 700–704 (1988).
Colón, W. & Roder, H. Kinetic intermediates in the formation of the cytochrome c molten globule. Nature Struct. Biol. 3, 1019–1025 (1996).
Elöve, G.A., Bhuyan, A.K. & Roder, H. Kinetic mechanism of cytochrome c folding: involvement of the heme and its ligands. Biochemistry 33, 6925–6935 (1994).
Ohgushi, M. & Wada, A. ‘Molten-globule state’: a compact form of globular proteins with mobile side-chains. FEBS Lett. 164, 21–24 (1983).
Kuroda, Y., Endo, S., Nagayama, K. & Wada, A. Stability of α-helices in a molten globule state of cytochrome c by hydrogen-deuterium exchange and two-dimensional NMR spectroscopy. J. Mol. Biol. 247, 682–688 (1995).
Jeng, M.F., Englander, S.W., Elöve, G.A., Wand, A.J. & Roder, H. Structural description of acid-denatured cytochrome c by hydrogen exchange and 2D NMR. Biochemistry 29, 10433–10437 (1990).
Dill, K.A. Dominant forces in protein folding. Biochemistry 29, 7133–7155 (1990).
Yee, D.P., Chan, H.S., Havel, T.F. & Dill, K.A. Does compactness induce secondary structure in proteins? A study of poly-alanine chains computed by distance geometry. J. Mol. Biol. 241, 557–573 (1994).
Marmorino, J.L. & Pielak, G.J. A native tertiary interaction stabilizes the A state of cytochrome c. Biochemistry 34, 3140–3143 (1995).
Marmorino, J.L., Lehti, M. & Pielak, G.J. Native tertiary structure in an A-state. J. Mol. Biol. 275, 379–388 (1998).
Kuroda, Y. Residual helical structure in the C-terminal fragment of cytochrome c. Biochemistry 32, 1219–1224 (1993).
Bai, Y., Sosnick, T.R., Mayne, L. & Englander, S.W. Protein folding intermediates: native-state hydrogen exchange. Science 269, 192–197 (1995).
Uversky, V.N. & Ptitsyn, O.B. ‘Partly folded’ state, a new equilibrium state of protein molecules: four-state guanidinium chloride-induced unfolding of β-lactamase at low temperature. Biochemistry 33, 2782–2791 (1994).
Acknowledgements
Supported by Grants-in-Aids for Scientific Research from the Ministry of Education, Science, Sports and Culture to S.T., K.I. and I.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akiyama, S., Takahashi, S., Ishimori, K. et al. Stepwise formation of α-helices during cytochrome c folding. Nat Struct Mol Biol 7, 514–520 (2000). https://doi.org/10.1038/75932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75932
This article is cited by
-
Treasurer’s comments on the financial position of the Biophysical Society of Japan
Biophysical Reviews (2020)
-
The aggregation of cytochrome C may be linked to its flexibility during refolding
3 Biotech (2016)
-
Microsecond protein dynamics observed at the single-molecule level
Nature Communications (2015)
-
Refolding of a membrane protein in a microfluidics reactor
European Biophysics Journal (2007)
-
Hierarchical folding of cytochrome c
Nature Structural Biology (2000)