Abstract
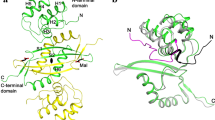
Laccase catalyses the oxidation of a variety of organic substrates coupled to the reduction of oxygen to water. It is widely believed to be the simplest representative of the ubiquitous blue multi-copper oxidase family. Laccase is implicated in a wide spectrum of biological activities and, in particular, plays a key role in morphogenesis, development and lignin metabolism in fungi and plants. The structure of laccase from the fungus Coprinus cinereus has been determined by X-ray crystallography at a resolution of 2.2 Å. Laccase is a monomer composed of three cupredoxin-like β-sandwich domains, similar to that found in ascorbate oxidase. In contrast to ascorbate oxidase, however, the mononuclear type-1 Cu site lacks the axial methionine ligand and so exhibits trigonal planar coordination, consistent with its elevated redox potential. Crucially, the structure is trapped in a Cu depleted form in which the putative type-2 Cu atom is completely absent, but in which the remaining type-1 and type-3 Cu sites display full occupancy. Type-2 Cu depletion has unexpected consequences for the coordination of the remaining type-3 Cu atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Xu, F. et al. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophysica Acta 1292, 303–311 (1996).
Xu, F. Oxidation of phenols, anilines and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry 35, 7608–7614 (1996).
Bao, W., O'Malley, D.M., Whetten, R. & Sederoff, R.R. A laccase associated with lignification in loblolly pine xylem. Science 260, 672–674 (1993).
Ander, P. & Eriksson, K.E. The importance of phenol oxidase activity in lignin degradation by the white rot fungus Sporotrichum pulverulentum. Arch. Microbiol. 109, 1–8 (1976).
Kersten, P.J., Kalyanaraman, B., Hammel, K.E., Reinhammar, B. & Kirk, T.K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem. J. 268, 475–480 (1990).
Leatham, G.F. & Stahmann, M.A. Studies on the laccase of Lentinus edodes: specificity, localization and assocition with the development of fruiting bodies. J. Gen. Microbiol. 125, 147–157 (1981).
Messerschmidt, A. Copper metalloenzymes. In Comprehensive Biological Catalysis Vol. III (ed. Sinnott, M.) 401–426 (Academic Press, London, 1997).
Messerschmidt, A. Multi-Copper Oxidases, (World Scientific, Singapore, 1997).
Malmström, B.C., Andréasson, L.E. & Reinhammar, R. Copper-containing oxidases and superoxide dismutase. In The Enzymes Vol. 12 (ed. Boyer, P.) 507–578 (Academic Press, New York, 1975):
Adman, E.T. Copper protein structures. Adv. Protein Chem. 42, 145–197 (1991).
Messerschmidt, A. et al. Refined crystal structure of ascorbate oxidase at 1.9Å resolution. J. Mol. Biol. 224, 179–205 (1992).
Zaitseva, I. et al. The X-ray structure of human serum ceruloplasmin at 3.1Å: nature of the copper centres. J. Biol. Inorg. Chem. 1, 15–23 (1996).
Holm, R.H., Kennepohl, P. & Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem Rev 96, 2239–2314 (1996).
Baker, E.N. Structure of Azurin from Alcaligenes denitrificans Refinement at 1.8 Å resolution and comparison of the two crystallographically independent molecules. J. Mol. Biol. 203, 1071–1095 (1988).
Collyer, C.A., Guss, J.M., Sugimura, Y., Yoshizaki, F. & Freeman, H.C. Crystal structure of plastocyanin from a green alga, Enteromorpha prolifera. J. Mol. Biol. 211, 617–632 (1990).
Murphy, M.E.P., Lindley, P.F. & Adman, E.T. Structural comparison of cupredoxin domains: domain recycling to construct proteins with novel functions. Protein Sci. 6, 761–770 (1997).
Reinhammar, B. In Copper Proteins and Copper Enzymes Vol. 3 (ed. Lontie, R.) 1–36 (CRC Press, Boca Raton, Florida, 1984).
Solomon, E.I., Sundaram, U.M. & Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 96, 2536–2605 (1996).
Messerschmidt, A. & Huber, R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin: modelling and structural relationships. Eur. J. Biochem. 187, 341–352 (1990).
Karlsson, B.G., Aasa, R., Malmström, B.G. & Lundberg, L.G. Rack-induced bonding in blue copper proteins: spectroscopic properties and reduction potential of azurin mutant Met-121 → Leu. FEBS Lett. 253, 99–102 (1989).
Guckert, J.A., Lowry, M.D. & Solomon, E.I. Electronic structure of the reduced blue copper active site: contributions to reduction potentials and geometry. J. Am. Chem. Soc. 117, 2817–2844 (1995).
Messerschmidt, A., Steigemann, W., Huber, R., Lang, G. & Kroneck, P.M.H. X-ray crystallographic characterisation of type-2-depleted ascorbate oxidase from zucchini. Eur. J. Biochem 209, 179–205 (1992).
LuBien, C.D. et al. Chemical and Spectroscopic proerties of the binuclear copper active site in Rhus laccase: direct conformation of a reduced binuclear Type 3 copper site in type 2 depleted laccase and intramolecular coupling of the type 3 to the type 1 and type 2 copper sites. J. Am. Chem. Soc. 103, 7014–7016 (1981).
Solomon, E.I., Machonkin, T.E. & Sundaram, U.M. Spectroscopy of multi-copper oxidases. in Multi-copper oxidases (ed. Messerschmidt, A.) 103–127 (World Scientific, Singapore, 1997).
Kau, L.-S., Spira-Solomon, D.J., Penner-Hahn, J.E., Hodgson, K.O. & Solomon, E.I. X-ray absorption edge determination of the oxidation state and coordination number of copper: application to the type 3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 109, 6433–6442 (1987).
Thurston, C.F. The structure and function of fungal laccases. Microbiology 140, 19–26 (1994).
Solomon, E.I. & Lowery, M.D. Electronic structure contributions to function in bioinorganic chemistry. Science 259, 1575–1581 (1993).
Cole, A.P., Root, D.E., Mukherjee, P., Solomon, E.I. & Stack, T.D.P. A Trinuclear Intermediate in the copper-mediated reduction of O2: four electrons from three coppers. Science 273, 1848–1850 (1996).
Graziani, M.T., Morpurgo, L., Rotilio, G. & Mondovi, B. Selective removal of type 2 copper from Rhus vernicifera laccase. FEBS Lett. 70, 87–(1976).
McMillin, D.R. & Eggleston, M.K. Bioinorganic chemistry of laccase. in Multi-copper oxidases (ed. Messerschmidt, A.) 129–166 (World Scientific, Singapore, 1997).
Reinhammar, B. & Oda, Y. Spectroscopic and catalytic properties of Rhus vernicifera laccase depleted in type 2 copper. J. Inorg. Biochem. 11, 115–127 (1979).
Ducros, V. et al. Crystallisation and preliminary X-ray analysis of the laccase from Coprinus cinereus. Acta. Crystallogr. D 53, 605–607 (1997).
Otwinowski, Z. Oscillation data reduction program. In Data collection and Processing: proceedings of the CCP4 study weekend (eds Sawyer, L., Issacs, N. & Bailey, S.) 56–62 (Science and Engineering Research Council, Daresbury, U.K, 1993).
Collaborative Computational Project Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D50, 760–763 (1994).
Navaza, J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A50, 157–163 (1994).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum likelihood method. Acta Crystallogr D 53, 240–255 (1997).
Buünger, A.T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Lamzin, V.S. & Wilson, K.S. Automated refinement of protein models. Acta Crystallogr. D49, 129–147 (1993).
Engh, R.A. & Huber, R. Accurate bond length and angle parameters for X-ray protein structure refinement. Acta Crystallogr. A 47, 392–400 (1991).
Ramachandran, G.N., Ramakrishnan, C. & Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol 7, 95–99 (1963).
Bernstein, F.C. et al. The protein data bank: a computer-based archival file for macromolecular structures. J. Mol. Biol 112, 535–542 (1977).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ducros, V., Brzozowski, A., Wilson, K. et al. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Mol Biol 5, 310–316 (1998). https://doi.org/10.1038/nsb0498-310
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0498-310
This article is cited by
-
Heterologous expression of Arabidopsis laccase2, laccase4 and peroxidase52 driven under developing xylem specific promoter DX15 improves saccharification in populus
Biotechnology for Biofuels and Bioproducts (2024)
-
A review of microbial laccase production and activity toward different biotechnological applications
Systems Microbiology and Biomanufacturing (2023)
-
Structural basis for monolignol oxidation by a maize laccase
Nature Plants (2020)
-
Crystal structures of multicopper oxidase CueO G304K mutant: structural basis of the increased laccase activity
Scientific Reports (2018)
-
Bioprospecting and biotechnological applications of fungal laccase
3 Biotech (2016)