Abstract
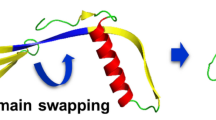
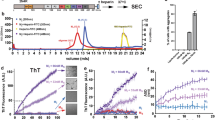
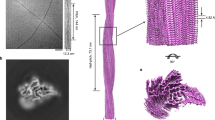
The crystal structure of human cystatin C, a protein with amyloidogenic properties and a potent inhibitor of cysteine proteases, reveals how the protein refolds to produce very tight two-fold symmetric dimers while retaining the secondary structure of the monomeric form. The dimerization occurs through three-dimensional domain swapping, a mechanism for forming oligomeric proteins. The reconstituted monomer-like domains are similar to chicken cystatin except for one inhibitory loop that unfolds to form the 'open interface' of the dimer. The structure explains the tendency of human cystatin C to dimerize and suggests a mechanism for its aggregation in the brain arteries of elderly people with amyloid angiopathy. A more severe 'conformational disease' is associated with the L68Q mutant of human cystatin C, which causes massive amyloidosis, cerebral hemorrhage and death in young adults. The structure of the three-dimensional domain-swapped dimers shows how the L68Q mutation destabilizes the monomers and makes the partially unfolded intermediate less unstable. Higher aggregates may arise through the three-dimensional domain-swapping mechanism occurring in an open-ended fashion in which partially unfolded molecules are linked into infinite chains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Grubb, A.O. Adv. Clin. Chem. 35, 63–99 (2000).
Turk, V. & Bode, W. FEBS Lett. 285, 213–219 (1991).
Olafsson, I. & Grubb, A. Amyloid Int. J. Exp. Clin. Invest. 7, 70–79 (2000).
Bode, W. et al. EMBO J. 7, 2593–2599 (1988).
Dieckmann, T. et al. J. Mol. Biol. 234, 1048–1059 (1993).
Engh, R.A. et al. J. Mol. Biol. 234, 1060–1069 (1993).
Stubbs, M.T. et al. EMBO J. 9, 1939–1947 (1990).
Martin, J.R. et al. J. Mol. Biol. 246, 331–343 (1995).
Chen, J.-M. et al. J. Biol. Chem. 272, 8090–8098 (1997).
Manoury, B. et al. Nature 369, 695–699 (1998).
Alvarez-Fernandez, M. et al. J. Biol. Chem. 274, 19195–19203 (1999).
Kozak, M. et al. Acta Crystallogr. D 55, 1939–1942 (1999).
Abrahamson, M. & Grubb, A. Proc. Natl. Acad. Sci. USA 91, 1416–1420 (1994).
Bjarnadottir, M. et al. Amyloid Int. J. Exp. Clin. Invest. 8, in the press (2001).
Bennett, M.J., Choe, S. & Eisenberg, D.S. Proc. Natl. Acad. Sci. USA 91, 3127–3131 (1994).
Schlunegger, M.P., Bennett, M.J. & Eisenberg, D. Adv. Protein Chem. 50, 61–122 (1997).
Bennett, M.J., Schlunegger, M.P. & Eisenberg, D. Protein Sci. 4, 2455–2468 (1995).
Ekiel, I. et al. J. Mol. Biol. 271, 266–277 (1997).
Rawlings, N.D. & Barrett, A.J. J. Mol. Evol. 30, 60–71 (1990).
Ni, J. et al. J. Biol. Chem. 273, 24797–24804 (1998).
Gerhartz, B., Ekiel, I. & Abrahamson, M. Biochemistry 37, 17309–17317 (1998).
Liu, Y., Hart, P.J., Schlunegger, M.P. & Eisenberg, D. Proc. Natl. Acad. Sci. USA 95, 3437–3442 (1998).
Ekiel, I. & Abrahamson, M. J. Biol. Chem. 271, 1314–1321 (1996).
Murray, A.J., Head, J.G., Barker, J.J. & Brady, R.L. Nature Struct. Biol. 5, 778–782 (1998).
Bonar, L., Cohen, A.S. & Skinner, M.M. Proc. Soc. Exp. Biol. Med. 131, 1373–1375 (1969).
Teplow, D.B. Amyloid Int. J. Exp. Clin. Invest. 5, 121–142 (1998).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Kissinger, C.R., Gehlhaar, D.K. & Fogel, D.B. Acta Crystallogr. D 55, 484–491 (1999).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Acknowledgements
The research of M.J. was supported by an International Research Scholar's award from the Howard Hughes Medical Institute. This research was sponsored by grants from the State Committee for Scientific Research, from the Swedish Medical Research Council, and from the A. Osterlund, A. Pahlsson and J. Kock Foundations. We thank V. Lindström, A.-C. Löfström, B. Gerhartz, and M. Alvarez-Fernandez for assistance with protein expression and purification, G. Bujacz for help with data collection, and Z. Otwinowski for advice on data processing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janowski, R., Kozak, M., Jankowska, E. et al. Human cystatin C, an amyloidogenic protein, dimerizes through three-dimensional domain swapping. Nat Struct Mol Biol 8, 316–320 (2001). https://doi.org/10.1038/86188
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/86188
This article is cited by
-
NAC blocks Cystatin C amyloid complex aggregation in a cell system and in skin of HCCAA patients
Nature Communications (2021)
-
Soluble Expression of Recombinant Human Cystatin C and Comparison of the Ni Column and Magnetic Bead Purification
The Protein Journal (2020)
-
The domain swapping of human cystatin C induced by synchrotron radiation
Scientific Reports (2019)
-
A five-residue motif for the design of domain swapping in proteins
Nature Communications (2019)
-
Stabilized Human Cystatin C Variant L47C/G69C Is a Better Reporter Than the Wild-Type Inhibitor for Characterizing the Thermodynamics of Binding to Cysteine Proteases
The Protein Journal (2019)