Abstract
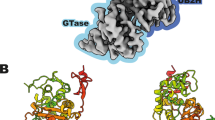
All β-lactam antibiotics exert their biological effects by interacting with a unique class of proteins, the penicillin-binding proteins (PBPs). These membrane proteins are involved in the biosynthesis of the murein or peptidoglycan, a mesh-like structure which completely surrounds the bacterial cell. Sequence similarities indicate that one domain of these proteins belongs to a large family of β-lactam-recognizing proteins, which includes the active-site serine β-lactamases. We here report the first three-dimensional crystal structure of a high molecular weight penicillin-binding protein, PBP2x of Streptococcus pneumoniae, at 3.5 Å resolution. The molecule has three domains, the central domain being a transpeptidase, which is a suitable target for antibiotic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hakenbeck, R., Briese, T. & Ellerbrok, H. Antibodies against the benzylpenicilloyl moiety as a probe for penicillin-binding proteins. Eur. J. Biochem. 157, 101–106 (1986).
Hakenbeck, R. & Kohiyama, M. Purification of penicillin-binding protein 3 from Streptococcus pneumoniae. Eur. J. Biochem. 127, 231–236 (1982).
Waxman, D.J. & Strominger, J.L. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu. Rev. Biochem. 52, 825–865 (1983).
Matsuhashi, M. in New Comprehensive Biochemistry. Bacterial Cell Wall Vol. 27, (eds J.-M. Ghuysen & R. Hakenbeck) 55–71 (Elsevier Science Publishers, Amsterdam 1994).
Spratt, B.G. Resistance to antibiotics mediated by target alterations. Science 264, 388–393 (1994).
Laible, G., Spratt, B.G. & Hakenbeck, R. Interspecies recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5, 1993–2002 (1991).
Laible, G. & Hakenbeck, R. Penicillin-binding proteins in β-lactam-resistant laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 1, 355–363 (1987).
Charlier, P. et al. Crystallization of a genetically engineered water-soluble primary penicillin target enzyme. The high molecular mass PBP2x of Streptococcus pneumoniae. J. Mol. Biol. 232, 1007–1009 (1993).
Lobkovsky, E. et al. Evolution of an enzyme activity: Crystallographic structure at 2- Å resolution of cephalosporinase from the ampCgene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 90, 11257–11261 (1993).
Kelly, J.A. & Kuzin, A.P. The refined Crystallographic structure of a DD-peptidase penicillin-target enzyme at 1.6 Å resolution. J. Mol. Biol. 254, 223–236 (1995).
Jamin, M., Damblon, C., Miller, S., Hakenbeck, R. & Frère, J.-M. Penicillin-binding protein 2x of Streptococcus pneumoniae: enzymic activities and interactions with β-lactams. Biochem. J. 292, 735–741 (1993).
Adachi, H., Ohta, T. & Matsuzawa, H. Site-directed mutants, at position 166, of RTEM-1 β-lactamase that form a stable acyl-enzyme intermediate with penicillin. J. Biol. Chem. 266, 3186–3191 (1991).
Bustos, J.F., Chait, B.T. & Tomasz, A. Structure of the peptide network of pneumococcal peptidoglycan. J. Biol. Chem. 262, 15400–15405 (1987).
Kuroki, R., Weaver, L.H. & Matthews, B.W. A covalent enzyme-substrate intermediate with saccharide distortion in a mutant T4 lysozyme. Science 262, 2030–2033 (1993).
Messerschmidt, A. & Pflugrath, J.W. Crystal orientation and X-ray pattern prediction routines for area-detector diffractometer systems in Macromolecular Crystallography. J. Appl. Crystallogr. 20, 306–315 (1987).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
CCP4, Collaborative Computing Project No.4, Acta Crystallogr. D50, 760–763 (1993).
Otwinowski, W. Isomorphous Replacement and Anomalous Scattering, Proceedings of the CCP4 Study Weekend. (eds Wolf, W., Evans, P.R. & Leslie, A.G.W.) 80–86 (SERC Daresbury Laboratory, Warrington, 1991).
Zhang, K.Y.J. & Main, P. The use of Sayre's equation with solvent flattening and histogram matching for phase extension and refinement of protein structures. Acta Crystallogr. A46, 377–381 (1990).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A47, 110–119 (1991).
Read, R.J., Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A42, 140–149 (1986).
Brünger, A.T. A system for X-ray Crystallography and NMR. X-PLOR Version 3.0. Yale Univ. Press, New Haven (1992).
Kraulis, P.J. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–286 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pares, S., Mouz, N., Pétillot, Y. et al. X-ray structure of Streptococcus pneumoniae PBP2x, a primary penicillin target enzyme. Nat Struct Mol Biol 3, 284–289 (1996). https://doi.org/10.1038/nsb0396-284
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0396-284
This article is cited by
-
PASTA sequence composition is a predictive tool for protein class identification
Amino Acids (2018)
-
Brevibacillus laterosporus isolated from the digestive tract of honeybees has high antimicrobial activity and promotes growth and productivity of honeybee’s colonies
Environmental Science and Pollution Research (2018)
-
Substitution of Alanine at Position 184 with Glutamic Acid in Escherichia coli PBP5 Ω-Like Loop Introduces a Moderate Cephalosporinase Activity
The Protein Journal (2018)
-
Distribution of PASTA domains in penicillin-binding proteins and serine/threonine kinases of Actinobacteria
The Journal of Antibiotics (2016)
-
Genome-scale analysis of the non-cultivable Treponema pallidum reveals extensive within-patient genetic variation
Nature Microbiology (2016)