Abstract
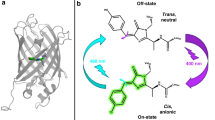
To understand how proteins translate the energy of sunlight into defined conformational changes, we have measured the photocycle reactions of photoactive yellow protein (PYP) using time-resolved step scan Fourier transform infrared (FTIR) spectroscopy. Global fit analysis yielded the same apparent time constants for the reactions of the chromophore, the protonation changes of protein side chains and the protein backbone motions, indicating that the light cycle reactions are synchronized. Changes in absorbance indicate that there are at least four intermediates (I1, I1′, I2, I2′). In the intermediate I1, the dark-state hydrogen bond from Glu 46 to the aromatic ring of the p-hydroxycinnamoyl chromophore is preserved, implying that the chromophore undergoes trans to cis isomerization by flipping, not the aromatic ring, but the thioester linkage with the protein. This excludes an I1 structural model proposed on the basis of time resolved Laue crystallography, but does agree with the cryotrapped structure of an I1 precursor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Meyer, T.E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium. Ectothiorhodospira halophila. Biochim. Biophys. Acta 806, 175–183 (1985).
Kort, R. et al. The xanthopsins; a new family of eubacterial blue-light photoreceptors. EMBO J. 15, 3209–3218 (1996).
Jiang, Z.Y. et al. Bacterial photoreceptor with similarity to photoactive yellow protein and plant phytochromes. Science 285, 406–409 (1999).
Sprenger, W.W., Hoff, W.D., Armitage, J.P. & Hellingwerf, K.J. The eubacterium Ectothiorhodospira halophila is negatively phototactic, with a wavelength dependence that fits the absorption spectrum of the photoactive yellow protein. J. Bacteriol. 175, 3096–3104 (1993).
Meyer, T.E., Yakali, E., Cusanovich, M.A. & Tollin, G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry 26, 418–423 (1987).
Baca, M. et al. Complete chemical structure of photoactive yellow protein: novel thioester-linked 4-hydroxycinnamyl chromophore and photocycle chemistry. Biochemistry 33, 14369–14377 (1994).
Hoff, W.D. et al. Thiol ester-linked p-coumaric acid as a new photoactive prosthetic group in a protein with rhodopsin-like photochemistry. Biochemistry 33, 13959–13962 (1994).
Meyer, T.E., Tollin, G., Hazzard, J.H. & Cusanovich, M.A. Photoactive yellow protein from the purple phototrophic bacterium, Ectothiorhodospira halophila. Quantum yield of photobleaching and effects of temperature, alcohols, glycerol, and sucrose on kinetics of photobleaching and recovery. Biophys. J. 56, 559–564 (1989).
Hoff, W.D. et al. Measurement and global analysis of the absorbance changes in the photocycle of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys. J. 67, 1691–1705 (1994).
Imamoto, Y., Kataoka, M. & Tokunaga, F. Photoreaction cycle of photoactive yellow protein from Ectothiorhodospira halophila studied by low-temperature spectroscopy. Biochemistry 35, 14047–14053 (1996).
Ujj, L. et al. New photocycle intermediates in the photoactive yellow protein from Ectothiorhodospira halophila; picosecond transient absorption spectroscopy. Biophys. J. 75, 406–412 (1998).
McRee, D.E., Meyer, T.E., Cusanovich, M.A., Parge, H.E. & Getzoff, E.D. Crystallographic characterization of a photoactive yellow protein with photochemistry similar to sensory rhodopsin. J. Biol. Chem. 261, 13850–13851 (1986).
Borgstahl, G.E.O., Williams, D.R. & Getzoff, E.D. 1.4 Å structure of photoactive yellow protein, a cytosolic photoreceptor: unusual fold, active site, and chromophore. Biochemistry 34, 6278–6287 (1995).
Düx, P. et al. Solution structure and backbone dynamics of the photoactive yellow protein. Biochemistry 37, 12689–12699 (1998).
Perman, B. et al. Energy transduction on the nanosecond time scale: early structural events in a xanthopsin photocycle. Science 279, 1946–1950 (1998).
Genick, U.K., Soltis, S.M., Kuhn, P., Canestrelli, I.L. & Getzoff, E.D. Structure at 0.85 Å resolution of an early protein photocycle intermediate. Nature 392, 206–209 (1998).
Genick, U.K. et al. Structure of a photocycle intermediate by millisecond time-resolved crystallography. Science 275, 1471–1475 (1997).
van Brederode, M.E., Hoff, W.D., van Stokkum, I.H.M., Groot, M.L. & Hellingwerf, K.J. Protein folding thermodynamics applied to the photocycle of the photoactive yellow protein. Biophys. J. 71, 365–380 (1996).
Rubinstenn, G. et al. Structural and dynamic changes of photoactive yellow protein during its photocycle in solution. Nature Struct. Biol. 5, 568–570 (1998).
Hoff, W.D. et al. Global conformational changes upon receptor stimulation in photoactive yellow protein. Biochemistry 38, 1009–1017 (1999).
Gerwert, K. Molecular reaction mechanisms of proteins as monitored by time-resolved FTIR spectroscopy. Current Opin. Struct. Biol. 3, 769–773 (1993).
Siebert, F. Infrared spectroscopy applied to biochemical and biological problems. Methods Enzymol. 246, 501–526 (1995).
Mathies, R.A. Biomolecular vibrational spectroscopy. Methods Enzymol. 246, 377–389 (1995).
Gerwert, K., Hess, B., Soppa, J. & Oesterhelt, D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 86, 4943–4947 (1989).
Xie, A., Hoff, W.D., Kroon, A.R. & Hellingwerf, K.J. Glu 46 donates a proton to the 4-hydroxycinnamate anion chromophore during the photocycle of photoactive yellow protein. Biochemistry 35, 14671–14678 (1996).
Hoff, W.D., Kwa, S.L.S., van Grondelle, R. & Hellingwerf, K.J. Low temperature absorbance and fluorescence spectroscopy of the photoactive yellow protein from Ectothiorhodospira halophila. Photochem. Photobiol. 56, 529–539 (1992).
Unno, M., Kumauchi, M., Sasaki, J., Tokunaga, F. & Yamauchi, S. Evidence for a protonated and cis configuration chromophore in the photobleached intermediate of photoactive yellow protein. J. Am. Chem. Soc. 122, 4233–4234 (2000).
Foerstendorf, H., Mummert, E., Schafer, E., Scheer, H. & Siebert, F. Fourier-transform infrared spectroscopy of phytochrome: difference spectra of the intermediates of the photoreactions. Biochemistry 35, 10793–10799 (1996).
Imamoto, Y. et al. Evidence for proton transfer from Glu-46 to the chromophore during the photocycle of photoactive yellow protein. J. Biol. Chem. 272, 12905–12908 (1997).
Zundel, G. Proton polarizability and proton transfer processes in hydrogen bonds and cation polarizabilities of other cation bonds; their importance to understand molecular processes in electrochemistry and biology. Trends Phys. Chem. 3, 129–156 (1992).
Devanathan, S. et al. Femtosecond spectroscopic observations of initial intermediates in the photocycle of the photoactive yellow protein from Ectothiorhodospira halophila. Biophys. J. 77, 1017–1023 (1999).
Srajer, V. et al. Photolysis of the carbon monoxide complex of myoglobin; nanosecond time-resolved crystallography. Science 274, 1726–1729 (1996).
Chu, K. et al. Structure of a ligand-binding intermediate in wild-type carbonmonoxy myoglobin. Nature 403, 921–923 (2000).
Genick, U.K. et al. Active site mutants implicate key residues for control of color and light cycle kinetics of photoactive yellow protein. Biochemistry 36, 8–14 (1997).
Brudler, R. et al. Coupling of hydrogen bonding to chromophore conformation and function in photoactive yellow protein. Biochemistry 39, 13478–13486 (2000).
Rammelsberg, R., Hessling, B., Chorongiewski, H. & Gerwert, K. Molecular reaction mechanisms of proteins monitored by nanosecond step-scan FT-IR difference spectroscopy. Appl. Spectrosc. 51, 558–562 (1997).
Hessling, B., Souvignier, G. & Gerwert, K. A model-independent approach to assigning bacteriorhodopsin's intramolecular reactions to photocycle intermediates. Biophys. J. 65, 1929–1941 (1993).
Acknowledgements
This work was supported by grants from the NIH to E.D.G. and the DFG to K.G. R.B. gratefully acknowledges a fellowship from the DFG.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brudler, R., Rammelsberg, R., Woo, T. et al. Structure of the I1 early intermediate of photoactive yellow protein by FTIR spectroscopy. Nat Struct Mol Biol 8, 265–270 (2001). https://doi.org/10.1038/85021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/85021
This article is cited by
-
The molecular pH-response mechanism of the plant light-stress sensor PsbS
Nature Communications (2021)
-
Confinement in crystal lattice alters entire photocycle pathway of the Photoactive Yellow Protein
Nature Communications (2020)
-
Volume-conserving trans–cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
Nature Chemistry (2013)