Abstract
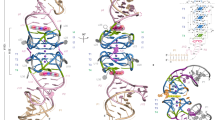
The crystal structure, at 2.8 Å resolution, of an RNA aptamer bound to bacteriophage MS2 coat protein has been determined. It provides an opportunity to compare the interactions of MS2 coat protein and wild type operator with those of an aptamer, whose secondary structure differs from the wild type RNA in having a three-base loop (compared to a tetraloop) and an additional base pair between this loop and the sequence-specific recognition element in the stem. The RNA binds in the same location on the coat protein as the wild type operator and maintains many of the same RNA–protein interactions. In order to achieve this, the RNA stem loop undergoes a concerted rearrangement of the 3′ side while leaving the 5′ side and the loop interactions largely unchanged, illustrating the ability of RNA to present similar molecular recognition surfaces from distinct primary and secondary structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Patel, D.J. et al. Structure, recognition and adaptive binding in RNA aptamer complexes. J. Mol. Biol. 272, 645–664 (1997)
Ye, X., Gorin, A., Ellington, A.D., & Patel, D.J. Deep penetration of an α-helix into a widened RNA major groove in the HIV-1 rev peptide-RNA aptamer complex. Nature Struct. Biol. 3, 1026–1033 (1996)
Schneider, D., Tuerk, C., & Gold, L. Selection of high affinity RNA ligands to the bacteriophage R17 coat protein. J. Mol. Biol. 228, 862–869 (1992)
Nieuwlandt, D., Wecker, M., & Gold, L. In vitro selection of RNA ligands to substance-P. Biochemistry 34, 5651–5659 (1995)
Connell, G.J., Illangesekare, M., & Yarus, M. 3 small ribooligonucleotides with specific arginine sites. Biochemistry 32, 5497–5502 (1993)
Sassanfar, M., & Szostak, J.W. An RNA motif that binds ATP. Nature 364, 550–555 (1993).
Ellington, A.D., & Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Jenison, R.D., Gill, S., Pardi, A. & Polishky, B. High resolution molecular discrimination by RNA. Science 263, 1425–1429– (1994).
Szostah, J.M., & Ellington, A.D. In The RNA world (eds. Gesteland, R.F., & Atkins, J.F.) 511–534 (Cold Spring Harbor Press, New York; 1993)
ValegÅrd, K., Murray, J.B., Stockley, P.G., Stonehouse, N.J., & Liljas, L. Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature 371, 623–626 (1994).
ValegÅrd, K. et al. The three-dimensional structures of two complexes between recombinant MS2 capsids and RNA operator fragments reveal sequence-specific protein–RNA interactions. J. Mol. Biol. 270, 724–738 (1997).
Witherell, G.W., Gott, J.M., & Uhlenbeck, O.C. Specific interaction between RNA phage coat protein and RNA. Prog. Nuc. Acid Res. 40, 185–220 (1991).
Talbot, S.J., Goodman, S., Bates, S.R.E., Fishwick, C.W.G., & Stockley, P.G. Use of synthetic oligoribonucleotides to probe RNA–protein interactions in the MS2 translational operator complex. Nucleic Acids Res. 18, 3521–3528 (1990).
Stockley, P.G. et al. Probing sequence-specific RNA recognition by the bacteriophage MS2 coat protein. Nucleic Acids Res. 23, 2512–2518 (1995).
ValegÅrd, K., Liljas, L., Fridborg, K., & Unge, T. The three-dimensional structure of the bacterial virus MS2 capsids. Nature 345, 36–41 (1990).
Peabody, D.S. The RNA binding site of bacteriophage MS2 coat protein. EMBO J. 12, 595–600 (1993).
Lim, F., & Peabody, D.S. Mutations that increase the affinity of a translational represser for RNA. Nucleic Acids Res. 22, 3748–3752 (1994).
Stockley, P.G., Stonehouse, N.J., & ValegÅrd, K. Molecular mechanism of RNA phage morphogenesis. Int. J. Biochem. 26, 1249–1260 (1994).
Lowary, P.T., & Uhlenbeck, O.C. An RNA mutation that increases the affinity of an RNA-protein interaction. Nucleic Acids Res. 15, 10483–10493 (1987).
Ni, C.-Z. et al. Crystal structure of the MS2 coat protein dimer: implications for RNA binding and virus assembly. Structure 3, 255–263 (1995).
Spingola, M., & Peabody, D.S. MS2 coat protein mutants which bind Qp RNA. Nucleic Acids Res. 25, 2808–2815 (1997).
Lim, F., Spingola, M., & Peabody, D.S. Altering the RNA binding specificity of a translational repressor. J. Biol. Chem. 269, 9006–9010 (1994).
Lim, F., Spingola, M., & Peabody, D.S. The RNA-binding site of bacteriophage Qβ coat protein. J. Biol. Chem. 271, 31839–31845 (1996).
Beckett, D., & Uhlenbeck, O.C. Ribonucleoprotein complexes of R17 coat protein and a translational operator analog. J. Mol. Biol. 204, 927–938 (1988).
Heus, H.A. RNA aptamers. Nature Struct. Biol. 4, 597–600 (1997).
Dieckmann, T., Butcher, S.E., Sassanfar, M., Szostak, J.W., & Feigon, J. Mutant ATP-binding RNA aptamers reveal the structural basis for ligand binding. J. Mol. Biol. 273, 467–478 (1997).
Uhlenbeck, O.C., Pardi, A., & Feigon, J. RNA structure comes of age. Cell 90, 833–840 (1997).
Cusack, S. 11 down and 9 to go. Nature Struct. Biol. 2, 824–831 (1995).
Oubridge, C., Ito, N., Evans, P.R., Teo, C.-H., & Nagai, K. Crystal structure at 1.92 Å resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature 372, 432–438 (1994).
Mastico, R.A., Talbot, S.J., & Stockley, P.G. Multiple presentation of foreign peptides on the surface of an RNA-free spherical bacteriophage capsid. J. Gen. Virol. 74, 541–548 (1993).
Stonehouse, N.J., & Stockley, P.G. Effects of amino acid substitution on the thermal stability of MS2 capsids lacking genomic RNA. FEBS Letts. 334, 355–359 (1993).
ValegÅrd, K., Unge, T., Montelius, I., Strandberg, B., & Fiers W. Purification, crystallization and preliminary X-ray data collection of the bacteriophage MS2. J. Mol. Biol. 190, 587–591 (1986).
Murray, J.B., Collier, A.K., & Arnold, J.R.P. A general purification procedure for chemically synthesised oligoribonucleotides. Anal. Biochem. 218, 177–184 (1994).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D50, 760–763 (1994).
Golmohammadi, R., ValegÅrd, K., Fridborg, K., & Liljas, L. The refined structure of bacteriophage MS2 at 2.8 Å resolution. J. Mol. Biol. 234, 620–639 (1993).
Kleywegt, G.J., & Jones, T.A. In From first map to final model (eds Bailey, S., Hubbard, R., & Waller, D.) 59–66 (EPSRC Daresbury Laboratory, Warrington, UK; 1994).
Jones, T.A., Zou, J.-Y, Cowan, S.W., & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 47, 110–119 (1991).
Brünger, A.T., Kuriyan, J., & Karplus, M. Crystallographic R factor refinement by molecular dynamics. Science 235, 458–460 (1987).
Engh, R.A., & Huber, R. Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr. 47, 392–400 (1991).
Parkinson, G., Vojtechovsky, J., Clowney, L., Brünger, AT., & Berman, H.M. New parameters for the refinement of nucleic acid-containing structures. Acta Crystallogr. D52, 57–64 (1996).
Brünger, A.T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992).
Jiang, J.-S., & Brünger, A.T. Protein hydration observed by X-ray diffraction: solvation properties of penicillopepsin and neuraminidase crystal structures. J. Mol. Biol. 243, 100–115 (1994).
Esnouf, R.M. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graphics 15, 133–138 (1997).
Ramakrishnan, C. & Ramachandran, G.N. Stereochemical criteria for polypeptide and protein chain conformations. II. Allowed conformations for a pair of peptide units. Biophys J. 5, 909–933 (1965).
Laskowski, R.A., MacArthur, M.W., Moss, D.S., & Thornton, J.M. PROCHECK: a program to check the stereochemistry of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Convery, M., Rowsell, S., Storehouse, N. et al. Crystal structure of an RNA aptamer–protein complex at 2.8 Å resolution. Nat Struct Mol Biol 5, 133–139 (1998). https://doi.org/10.1038/nsb0298-133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb0298-133
This article is cited by
-
A comparative analysis of cell surface targeting aptamers
Nature Communications (2021)
-
RNA origami design tools enable cotranscriptional folding of kilobase-sized nanoscaffolds
Nature Chemistry (2021)
-
Branched kissing loops for the construction of diverse RNA homooligomeric nanostructures
Nature Chemistry (2020)
-
In situ structures of the genome and genome-delivery apparatus in a single-stranded RNA virus
Nature (2017)
-
Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ
Nature Communications (2016)