Abstract
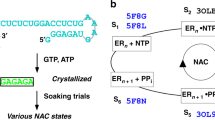
RNA cleavage by the Tetrahymena ribozyme requires recognition of the reaction–site helix by the catalytic apparatus. This binding can occur in several registers, each of which results in reaction at a different nucleotide in the helix. We now identify commensurate sets of 2′–hydroxyl interactions on both strands of the reaction–site helix that account for its translocation into alternative binding registers. These results indicate that the ribozyme has a relatively rigid substrate–binding pocket into which the helix can bind in different alignments. A similar mechanism of reaction site recognition is proposed to occur during intron circularization and ribozyme polymerase activity. Translocation of the reaction site duplex provides an example of structural heterogeneity in packing of helices during the teritary folding of RNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cech, T.R., Herschlag, D., Piccirilli, J.A. & Pyle, A.M. RNA catalysis by a group I ribozyme: developing a model for transition state stabilization. J. biol. Chem. 267, 17479–17482 (1992).
Zaug, A.J., Grosshans, C.A. & Cech, T.R. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry 27, 8924–8931 (1988).
Herschlag, D. & Cech, T.R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 1. Kinetic description of the reaction of an RNA substrate complementary to the active site. Biochemistry 29, 10159–10171 (1990).
Herschlag, D. Evidence for processivity and two-step binding of the RNA substrate from studies of J1/2 mutants of the Tetrahymena ribozyme. Biochemistry 31, 1386–1399 (1992).
Bevilacqua, P.C., Kierzek, R., Johnson, K.A. & Turner, D.H. Dynamics of ribozyme binding of substrate revealed by fluorescence-detected stopped-flow methods. Science 258, 1355–1358 (1992).
Been, M.D. & Cech, T.R. One binding site determines sequence specificity of Tetrahymena pre-rRNA self-splicing, trans-splicing and RNA enzyme activity. Cell 47, 207–216 (1986).
Waring, R.B., Towner, P., Minter, S.J. & Davies, R.W. Splice-site selection by a self-splicing RNA of Tetrahymena. Nature 321, 133–139 (1986).
Pyle, A.M. & Cech, T.R. Ribozyme recognition of RNA by tertiary interactions with specific ribose 2′-OH groups. Nature 350, 628–631 (1991).
Bevilacqua, P.C. & Turner, D.H. Comparison of binding of mixed ribose-deoxyribose analogues of CUCU to a ribozyme and to GGAGAA by equilibrium dialysis: evidence for ribozyme specific interaction with 2′-OH groups. Biochemistry 30, 10632–10640 (1991).
Herschlag, D., Eckstein, F. & Cech, T.R. Contributions of 2′-hydroxyl groups of the RNA substrate to binding and catalysis by the Tetrahymena ribozyme. An energetic picture of an active site composed of RNA. Biochemistry 32, 8299–8311 (1993).
Strobel, S.A. & Cech, T.R. Tertiary interactions with the internal guide sequence mediate docking of the P1 helix into the catalytic core of the Tetrahymena ribozyme. Biochemistry (in the press).
Been, M.D. & Cech, T.R. Selection of circularization sites in a group I IVS RNA requires multiple alignments of an internal template-like sequence. Cell 50, 951–961 (1987).
Doudna, J.A., Cormack, B.P. & Szostak, J.W. RNA structure, not sequence, determines the 5′ splice-site specificity of a group I intron. Proc. natn. Acad. Sci. U.S.A. 86, 7402–7406 (1989).
Moore, M.J. & Sharp, P.A. Site-specific modification of pre-mRNA: the 2′-hydroxyl groups at the splice sites. Science 256, 992–997 (1992).
Herschlag, D., Piccirilli, J.A. & Cech, T.R. Ribozyme-catalyzed and non-enzymatic reactions of phosphate diesters: rate effects upon substitution of sulfur for a nonbridging phosphoryl oxygen atom. Biochemistry 30, 4844–4854 (1991).
Barfod, E.T. & Cech, T.R. The conserved U·G pair in the 5′ splice site duplex of a group I intron is required in the first but not the second step of self-splicing. Molec. cell. Biol. 9, 3657–3666 (1989).
Young, B., Herschlag, D. & Cech, T.R. Mutations in a nonconserved sequence of the Tetrahymena ribozyme increase activity and specificity. Cell 67, 1007–1019 (1991).
Downs, W.D. & Cech, T.R. An ultraviolet-inducible adenosine-adenosine crosslink reflects the catalytic structure of the Tetrahymena ribozyme. Biochemistry 29, 5605–5613 (1990).
Doudna, J.A. & Szostak, J.W. RNA-catalysed synthesis of complementary-strand RNA. Nature 339, 519–522 (1989).
Been, M.D. & Cech, T.R. RNA as an RNA polymerase: net elongation of an RNA primer catalyzed by the Tetrahymena ribozyme. Science 239, 1412–1416 (1988).
Doudna, J.A., Couture, S. & Szostak, J.W. A multisubunit ribozyme that is a catalyst of and template for complementary strand RNA synthesis. Science 251, 1605–1608 (1991).
Holbrook, S.R., Cheong, C., Tinoco, I. & Kim, S.H. Crystal structure of an RNA double helix incorporating a track of non-Watson-Crick base pairs. Nature 353, 579–581 (1992).
Michel, F., Hanna, M., Green, R., Bartel, D.P. & Szostak, J.W. The guanosine binding site of the Tetrahymena ribozyme. Nature 342, 391–395 (1989).
Yarus, M., Illangesekare, M. & Christian, E. An axial binding site in the Tetrahymena precursor RNA. J. molec. Biol. 222, 995–1012 (1991).
Pyle, A.M., Murphy, F.L. & Cech, T.R. RNA substrate binding site in the catalytic core of the Tetrahymena ribozyme. Nature 358, 123–128 (1992).
Michel, F. & Westhof, E. Modelling of the three-dimensional architecture of Group I catalytic introns based on comparative sequence analysis. J. molec. Biol. 216, 585–610 (1990).
Wang, J.F. & Cech, T.R. Tertiary structure around the guanosine-binding site of the Tetrahymena ribozyme. Science 256, 526–529 (1992).
Wang, J.F., Downs, W.D. & Cech, T.R. Movement of the guide sequence during RNA catalysis by a group I ribozyme. Science 260, 504–508 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Strobel, S., Cech, T. Translocation of an RNA duplex on a ribozyme. Nat Struct Mol Biol 1, 13–17 (1994). https://doi.org/10.1038/nsb0194-13
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0194-13