Abstract
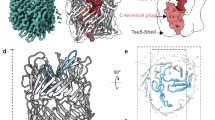
Pseudomonas aeruginosa is an opportunistic bacterial pathogen. One of its major toxins, ExoS, is translocated into eukaryotic cells by a type III secretion pathway. ExoS is a dual function enzyme that affects two different Ras-related GTP binding proteins. The C-terminus inactivates Ras through ADP ribosylation, while the N-terminus inactivates Rho proteins through its GTPase activating protein (GAP) activity. Here we have determined the three-dimensional structure of a complex between Rac and the GAP domain of ExoS in the presence of GDP and AlF3. Composed of ∼130 residues, this ExoS domain is the smallest GAP hitherto described. The GAP domain of ExoS is an all-helical protein with no obvious structural homology, and thus no recognizable evolutionary relationship, with the eukaryotic RhoGAP or RasGAP fold. Similar to other GAPs, ExoS downregulates Rac using an arginine finger to stabilize the transition state of the GTPase reaction, but the details of the ExoS–Rac interaction are unique. Considering the intrinsic resistance of P. aeruginosa to antibiotics, this might open up a new avenue towards blocking its pathogenicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Bourne H.R., Sanders D.A. & McCormick F. Nature 348, 125–132 (1990).
Bourne H.R., Sanders D.A. & McCormick F. Nature 349, 117–127 (1991).
Aktories, K. J. Clin. Invest. 99, 827–829 (1997).
Hardt, W.D., Chen, L.M., Schuebel, K.E., Bustelo, X.R. & Galán, J.E. Cell 93, 815–826 (1998).
Goehring, U.M., Schmidt, G., Pederson, K.J., Aktories, K. & Barbieri, J.T. J. Biol. Chem. 274, 36369–36372 (1999).
Fu, Y.X. & Galán, J.E. Nature 401, 293–297 (1999).
Pawel-Rammingen, U. et al. Mol. Microbiol. 36, 737–748 (2000).
Scheffzek, K., Stephan, I., Jensen, O.N., Illenberger, D. & Gierschik, P. Nature Struct. Biol. 7, 122–126 (2000).
Scheffzek, K. et al. Science 277, 333–338 (1997).
Rittinger, K., Walker, P.A., Eccleston, J.F., Smerdon, S.J. & Gamblin, S.J. Nature 389, 758–762 (1997).
Nassar, N., Hoffman, G.R., Manor, D., Clardy, J.C. & Cerione, R.A. Nature Struct. Biol. 5, 1047–1052 (1998).
Rak, A. et al. EMBO J. 19, 5105–5113 (2000).
Hillig, R.C. et al. Mol. Cell 3, 781–791 (1999).
Goldberg, J. Cell 96, 893–902 (1999).
Scheffzek, K., Ahmadian, M.R. & Wittinghofer, A. Trends Biochem. Sci. 23, 257–262 (1998).
Tesmer, J.J., Berman, D.M., Gilman, A.G. & Sprang, S.R. Cell 89, 251–261 (1997).
Holm, L. & Sander, C. Nucleic Acids Res. 27, 244–247 (1999).
Graham, D.L., Eccleston, J.F. & Lowe, P.N. Biochemistry 38, 985–991 (1999).
Ahmadian, M.R., Stege, P., Scheffzek, K. & Wittinghofer, A. Nature Struct. Biol. 4, 686–689 (1997).
Wittinghofer, A. Curr. Biol. 7, R682–R685 (1997).
Schlichting, I. & Reinstein, J. Nature Struct. Biol. 6, 721–723 (1999).
Caron, E. & Hall, A. Science 282, 1717–1721 (1998).
Massol, P., Montcourrier, P., Guillemot, J.C. & Chavrier, P. EMBO J. 17, 6219–6229 (1998).
Otwinowski, Z. & Minor, D.L. Methods Enzymol. 276, 307–326 (1997).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Perrakis, A., Morris, R. & Lamzin, V.S. Nature Struct. Biol. 6, 458–463 (1999).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard . Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Laskowski, R.A., MacArthur, M.W. & Moss, D.S & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Hooft R.W.W., Vriend G., Sander C. & Abola E.E., Nature 381, 272 (1996).
Kabsch, W. & Sander, C. Biopolymers 22, 2577–2637 (1983).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Acknowledgements
We thank S. McSweeny, A. Perrakis and ESRF for beam-time allocation and help during data collection. We additionally thank E. Carrier and A. Gerhards for technical assistance, M. Hess for help with the figures, K. Scheffzek for carefully reading the manuscript, and R. Schebaum for secretarial assistance. J.T.B. was supported by the NIH, A.W. by the DFG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Würtele, M., Wolf, E., Pederson, K. et al. How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nat Struct Mol Biol 8, 23–26 (2001). https://doi.org/10.1038/83007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83007
This article is cited by
-
Computational approach to attenuate virulence of Pseudomonas aeruginosa through bioinspired silver nanoparticles
3 Biotech (2022)
-
Bacterial membrane vesicles transport their DNA cargo into host cells
Scientific Reports (2017)
-
The type III secretion system of Pseudomonas aeruginosa: infection by injection
Nature Reviews Microbiology (2009)
-
The evolutionary conundrum of pathogen mimicry
Nature Reviews Microbiology (2009)
-
Protein delivery into eukaryotic cells by type III secretion machines
Nature (2006)