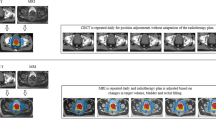
Key Points
-
Brachytherapy and brachytherapy boost with low-dose-rate brachytherapy (LDR-BT) or high-dose-rate (HDR)-BT can be used as first-line therapies in the management of prostate cancer patients of all National Comprehensive Cancer Network (NCCN)-defined risk groups
-
LDR-BT, consisting of a single implant, typically uses 125I or 103Pd; by contrast, HDR-BT consists of 1–3 implants and uses 192Ir
-
Benefits of HDR-BT over LDR-BT include the ability to use the same source for other cancers, lower operator dependence, and fewer acute irritative symptoms
-
Benefits of LDR-BT include more favourable scheduling logistics, lower initial capital equipment costs, non-requirement of a shielded room, completion in a single implant, and more robust data from clinical trials
-
Outcomes of HDR-BT and LDR-BT are similar to those of other treatment options, including external beam radiotherapy (EBRT) and surgery, and brachytherapy can also be used in combination with EBRT in intermediate-risk and high-risk disease
-
Severe toxicities of HDR-BT and LDR-BT are rare, although the rate of urethral stricture is increased when brachytherapy boost is performed; incontinence is not associated with any radiotherapy modality
Abstract
Brachytherapy (BT), using low-dose-rate (LDR) permanent seed implantation or high-dose-rate (HDR) temporary source implantation, is an acceptable treatment option for select patients with prostate cancer of any risk group. The benefits of HDR-BT over LDR-BT include the ability to use the same source for other cancers, lower operator dependence, and — typically — fewer acute irritative symptoms. By contrast, the benefits of LDR-BT include more favourable scheduling logistics, lower initial capital equipment costs, no need for a shielded room, completion in a single implant, and more robust data from clinical trials. Prospective reports comparing HDR-BT and LDR-BT to each other or to other treatment options (such as external beam radiotherapy (EBRT) or surgery) suggest similar outcomes. The 5-year freedom from biochemical failure rates for patients with low-risk, intermediate-risk, and high-risk disease are >85%, 69–97%, and 63–80%, respectively. Brachytherapy with EBRT (versus brachytherapy alone) is an appropriate approach in select patients with intermediate-risk and high-risk disease. The 10-year rates of overall survival, distant metastasis, and cancer-specific mortality are >85%, <10%, and <5%, respectively. Grade 3–4 toxicities associated with HDR-BT and LDR-BT are rare, at <4% in most series, and quality of life is improved in patients who receive brachytherapy compared with those who undergo surgery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Global Burden of Disease Cancer, C. et al. The global burden of cancer 2013. JAMA Oncol. 1, 505–527 (2015).
Cahlon, O. et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 71, 330–337 (2008).
Mohler, J. L. et al. Prostate cancer, version 1.2016. J. Natl Compr. Canc. Netw. 14, 19–30 (2016).
Zaorsky, N. G. et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat. Rev. 48, 50–60 (2016).
Donovan, J. L. et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 375, 1425–1437 (2016).
Hamdy, F. C. et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med. 375, 1415–1424 (2016).
Shen, X. et al. Comparative effectiveness research for prostate cancer radiation therapy: current status and future directions. Future Oncol. 8, 37–54 (2012).
Davis, B. J. et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy 11, 6–19 (2012).
Yamada, Y. et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 11, 20–32 (2012).
Salembier, C. et al. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother. Oncol. 83, 3–10 (2007).
Hoskin, P. J. et al. GEC/ESTRO recommendations on high dose rate afterloading brachytherapy for localised prostate cancer: an update. Radiother. Oncol. 107, 325–332 (2013).
Case, J. T. The early history of radium therapy and the American Radium Society. Am. J. Roentgenol. Radium Ther. Nucl. Med. 82, 574–585 (1959).
Zeitlin, S. I., Sherman, J., Raboy, A., Lederman, G. & Albert, P. High dose combination radiotherapy for the treatment of localized prostate cancer. J. Urol. 160, 91–96 (1998).
Garzotto, M. & Fair, W. R. Historical perspective on prostate brachytherapy. J. Endourol. 14, 315–318 (2000).
Lederman, M. The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol. Biol. Phys. 7, 639–648 (1981).
Young, H. H. The use of radium and the punch operation in desperate cases of enlarged prostate. Ann. Surg. 65, 633–641 (1917).
Deming, C. L. Results in one hundred cases of cancer of prostate and seminal vesicles treated with radium. Surg. Gynecol. Obstet. 34, 99–118 (1922).
Flocks, R. H., Kerr, H. D., Elkins, H. B. & Culp, D. A. The treatment of carcinoma of the prostate by interstitial radiation with radioactive gold (Au198); a follow-up report. J. Urol. 71, 628–633 (1954).
Flocks, R. H. Interstitial irradiation therapy with a solution of Au198 as part of combination therapy for prostatic carcinoma. J. Nucl. Med. 5, 691–705 (1964).
Bagshaw, M. A., Kaplan, H. S. & Sagerman, R. H. Linear accelerator supervoltage VII: carcinoma of the prostate. Radiology 85, 121–129 (1965).
Whitmore, W. F. Jr, Hilaris, B. & Grabstald, H. Retropubic implantation to iodine 125 in the treatment of prostatic cancer. J. Urol. 108, 918–920 (1972).
Hilaris, B. S., Whitmore, W. F. Jr, Batata, M. A. & Grabstald, H. Radiation therapy and pelvic node dissection in the management of cancer of the prostate. Am. J. Roentgenol. Radium Ther. Nucl. Med. 121, 832–838 (1974).
Zaorsky, N. G. et al. A paradigm shift from anatomic to functional and molecular imaging in the detection of recurrent prostate cancer. Future Oncol. 10, 457–474 (2014).
Whitmore, W. F. Jr, Hilaris, B., Grabstald, H. & Batata, M. Implantation of 125I in prostatic cancer. Surg. Clin. North Am. 54, 887–895 (1974).
Whitmore, W. F. Jr, Hilaris, B. & Grabstald, H. Retropubic implantation of iodine 125 in the treatment of prostatic cancer. Trans. Am. Assoc. Genitourin. Surg. 64, 55–57 (1972).
Batata, M. A. et al. Radiation therapy in adenocarcinoma of the prostate with pelvic lymph node involvement on lymphadenectomy. Int. J. Radiat. Oncol. 6, 149–153 (1980).
Whitmore, W. F. Jr. Interstitial radiation therapy for carcinoma of the prostate. Prostate 1, 157 (1980).
Zelefsky, M. J. & Whitmore, W. F. Jr. Long-term results of retropubic permanent 125Iodine implantation of the prostate for clinically localized prostatic cancer. J. Urol. 158, 23–30 (1997).
Ragde, H. et al. Interstitial iodine-125 radiation without adjuvant therapy in the treatment of clinically localized prostate carcinoma. Cancer 80, 442–453 (1997).
Gottesman, J. E., Tesh, D. G. & Weissman, W. D. Failure of open radioactive 125Iodine implantation to control localized prostate cancer: a study of 41 patients. J. Urol. 146, 1317–1320 (1991).
Kuban, D. A., el-Mahdi, A. M. & Schellhammer, P. F. I-125 interstitial implantation for prostate cancer. What have we learned 10 years later? Cancer 63, 2415–2420 (1989).
Holm, H. H., Juul, N., Pedersen, J. F., Hansen, H. & Stroyer, I. Transperineal 125iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. 1983. J. Urol. 167, 985–989 (2002).
Blasko, J. C. et al. Should brachytherapy be considered a therapeutic option in localized prostate cancer? Urol. Clin. North Am. 23, 633–650 (1996).
Heysek, R. V. Modern brachytherapy for treatment of prostate cancer. Cancer Control 14, 238–243 (2007).
Zaorsky, N. G. et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 10, 565–579 (2013).
Thompson, I. et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J. Urol. 177, 2106–2131 (2007).
Tapen, E. M. et al. Reduction of radioactive seed embolization to the lung following prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 42, 1063–1067 (1998).
Schwartz, D. J. et al. Radiation exposure to operating room personnel during transperineal interstitial permanent prostate brachytherapy. Brachytherapy 2, 98–102 (2003).
Mate, T. P., Gottesman, J. E., Hatton, J., Gribble, M. & Van Hollebeke, L. High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. Int. J. Radiat. Oncol. Biol. Phys. 41, 525–533 (1998).
McNeal, J. E., Redwine, E. A., Freiha, F. S. & Stamey, T. A. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am. J. Surg. Pathol. 12, 897–906 (1988).
McNeal, J. E. et al. Patterns of progression in prostatic carcinoma. Lancet 1, 60–63 (1986).
Stromberg, J. et al. Ultrasound-guided high dose rate conformal brachytherapy boost in prostate cancer: treatment description and preliminary results of a phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 33, 161–171 (1995).
Kovacs, G. et al. Prostate preservation by combined external beam and HDR brachytherapy in nodal negative prostate cancer. Strahlenther. Onkol. 175 (Suppl. 2), 87–88 (1999).
Duchesne, G. M., Williams, S. G., Das, R. & Tai, K. H. Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer: mature prospective phase I/II study results. Radiother. Oncol. 84, 128–134 (2007).
Galalae, R. M. et al. Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther. Onkol. 182, 135–141 (2006).
Kalkner, K. M. et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 46, 909–917 (2007).
Hsu, I. C. et al. Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int. J. Radiat. Oncol. Biol. Phys. 78, 751–758 (2010).
Martinez, A. et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J. Urol. 169, 974–980 (2003).
Martinez, A. A. et al. High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am. J. Clin. Oncol. 33, 481–488 (2010).
Martinez-Monge, R. et al. External-beam radiation therapy and high-dose rate brachytherapy combined with long-term androgen deprivation therapy in high and very high prostate cancer: preliminary data on clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 82, e469–476 (2012).
Morton, G. et al. Is single fraction 15 Gy the preferred high dose-rate brachytherapy boost dose for prostate cancer? Radiother. Oncol. 100, 463–467 (2011).
Myers, M. A. et al. Phase I/II trial of single-fraction high-dose-rate brachytherapy-boosted hypofractionated intensity-modulated radiation therapy for localized adenocarcinoma of the prostate. Brachytherapy 11, 292–298 (2012).
Vargas, C. E. et al. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: results of a phase I to II study. Int. J. Radiat. Oncol. Biol. Phys. 66, 416–423 (2006).
Hoskin, P. J. et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 103, 217–222 (2012).
Zaorsky, N. G., Den, R. B., Doyle, L. A., Dicker, A. P. & Hurwitz, M. D. Combining theoretical potential and advanced technology in high-dose rate brachytherapy boost therapy for prostate cancer. Expert Rev. Med. Devices 10, 751–763 (2013).
Zaorsky, N. G. et al. High dose rate brachytherapy boost for prostate cancer: a systematic review. Cancer Treat. Rev. 40, 414–425 (2014).
Yoshioka, Y. et al. High-dose-rate interstitial brachytherapy as a monotherapy for localized prostate cancer: treatment description and preliminary results of a phase I/II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 48, 675–681 (2000).
Yoshioka, Y., Yoshida, K., Yamazaki, H., Nonomura, N. & Ogawa, K. The emerging role of high-dose-rate (HDR) brachytherapy as monotherapy for prostate cancer. J. Radiat. Res. 54, 781–788 (2013).
Yoshioka, Y. et al. Nationwide, multicenter, retrospective study on high-dose-rate brachytherapy as monotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 97, 952–961 (2017).
Yoshioka, Y. et al. High-dose-rate brachytherapy as monotherapy for intermediate- and high-risk prostate cancer: clinical results for a median 8-year follow-up. Int. J. Radiat. Oncol. Biol. Phys. 94, 675–682 (2016).
Martinez, A. A. et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int. J. Radiat. Oncol. Biol. Phys. 49, 61–69 (2001).
Morton, G. C. The emerging role of high-dose-rate brachytherapy for prostate cancer. Clin. Oncol. 17, 219–227 (2005).
Zaorsky, N. G., Doyle, L. A., Hurwitz, M. D., Dicker, A. P. & Den, R. B. Do theoretical potential and advanced technology justify the use of high-dose rate brachytherapy as monotherapy for prostate cancer? Expert Rev. Anticancer Ther. 14, 39–50 (2014).
Martin, J. M. et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 120, 2114–2121 (2014).
Glaser, S. M. et al. Brachytherapy boost for prostate cancer: trends in care and survival outcomes. Brachytherapy 16, 330–341 (2017).
Zaorsky, N. G. et al. What are medical students in the United States learning about radiation oncology? Results of a multi-institutional survey. Int. J. Radiat. Oncol. Biol. Phys. 94, 235–242 (2016).
Zaorsky, N. G. et al. Impact of a radiation oncology elective on the careers of young physicians: update on a prospective cohort study. Int. J. Radiat. Oncol. Biol. Phys. 86, 214–215 (2013).
Gondi, V. et al. Results of the 2005–2008 Association of Residents in Radiation Oncology survey of chief residents in the United States: clinical training and resident working conditions. Int. J. Radiat. Oncol. Biol. Phys. 81, 1120–1127 (2011).
Nabavizadeh, N. et al. Results of the 2013–2015 Association of Residents in Radiation Oncology Survey of Chief Residents in the United States. Int. J. Radiat. Oncol. Biol. Phys. 94, 228–234 (2016).
Compton, J. J. et al. Resident-reported brachytherapy experience in ACGME-accredited radiation oncology training programs. Brachytherapy 12, 622–627 (2013).
Battermann, J. J. & van Es, C. A. The learning curve in prostate seed implantation. Cancer Radiother. 4 Suppl 1, 119s–122s (2000).
Lee, W. R., deGuzman, A. F., Bare, R. L., Marshall, M. G. & McCullough, D. L. Postimplant analysis of transperineal interstitial permanent prostate brachytherapy: evidence for a learning curve in the first year at a single institution. Int. J. Radiat. Oncol. Biol. Phys. 46, 83–88 (2000).
Acher, P. et al. Permanent prostate brachytherapy: dosimetric results and analysis of a learning curve with a dynamic dose-feedback technique. Int. J. Radiat. Oncol. Biol. Phys. 65, 694–698 (2006).
Avkshtol, V. et al. A comparison of robotic arm versus gantry linear accelerator stereotactic body radiation therapy for prostate cancer. Res. Rep. Urol. 8, 145–158 (2016).
Zaorsky, N. G., Studenski, M. T., Dicker, A. P., Gomella, L. & Den, R. B. Stereotactic body radiation therapy for prostate cancer: is the technology ready to be the standard of care? Cancer Treat. Rev. 39, 212–218 (2013).
Zaorsky, N. G., Hurwitz, M. D., Dicker, A. P., Showalter, T. N. & Den, R. B. Is robotic arm stereotactic body radiation therapy “virtual high dose ratebrachytherapy” for prostate cancer? An analysis of comparative effectiveness using published data [corrected]. Expert Rev. Med. Devices 12, 317–327 (2015).
Zaorsky, N. G., Li, T., Devarajan, K., Horwitz, E. M. & Buyyounouski, M. K. Assessment of the American Joint Committee on Cancer staging (sixth and seventh editions) for clinically localized prostate cancer treated with external beam radiotherapy and comparison with the National Comprehensive Cancer Network risk-stratification method. Cancer 118, 5535–5543 (2012).
Zaorsky, N. G. et al. Causes of death among cancer patients. Ann. Oncol. 28, 400–407 (2017).
Kleinmann, N. et al. The effect of ethnicity and sexual preference on prostate-cancer-related quality of life. Nat. Rev. Urol. 9, 258–265 (2012).
Astrom, L., Pedersen, D., Mercke, C., Holmang, S. & Johansson, K. A. Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother. Oncol. 74, 157–161 (2005).
Ghilezan, M. et al. 10-year results in 1577 intermediate/high risk prostate cancer patients treated with external beam RT (EBRT) and hypofractionated high dose rate (HDR) brachytherapy boost. Int. J. Rad Biol. Phys. 69, S83–84 (2007).
Kaprealian, T. et al. High-dose-rate brachytherapy boost for prostate cancer: comparison of two different fractionation schemes. Int. J. Radiat. Oncol. Biol. Phys. 82, 222–227 (2012).
Kestin, L. L. et al. Pathologic evidence of dose-response and dose-volume relationships for prostate cancer treated with combined external beam radiotherapy and high-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 54, 107–118 (2002).
Mohammed, N. et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 82, 204–212 (2012).
Prada, P. J. et al. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 years of experience. BJU Int. 109, 1787–1793 (2012).
Vicini, F., Vargas, C., Gustafson, G., Edmundson, G. & Martinez, A. High dose rate brachytherapy in the treatment of prostate cancer. World J. Urol. 21, 220–228 (2003).
Vicini, F. A., Vargas, C., Edmundson, G., Kestin, L. & Martinez, A. The role of high-dose rate brachytherapy in locally advanced prostate cancer. Semin. Radiat. Oncol. 13, 98–108 (2003).
Shen, X., Keith, S. W., Mishra, M. V., Dicker, A. P. & Showalter, T. N. The impact of brachytherapy on prostate cancer-specific mortality for definitive radiation therapy of high-grade prostate cancer: a population-based analysis. Int. J. Radiat. Oncol. Biol. Phys. 83, 1154–1159 (2012).
Morris, W. J. et al. ASCENDE-RT: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. J. Clin. Oncol. 33 (Suppl 7) abstr. 03 (2015).
Yamoah, K. et al. Large prostate gland size is not a contraindication to low-dose-rate brachytherapy for prostate adenocarcinoma. Brachytherapy 13, 456–464 (2014).
Zaorsky, N. G., Trabulsi, E. J., Lin, J. & Den, R. B. Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin. Oncol. 40, 308–321 (2013).
Zumsteg, Z. S. et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur. Urol. 64, 895–902 (2013).
D'Amico, A. V., Chen, M. H., Renshaw, A. A., Loffredo, M. & Kantoff, P. W. Androgen suppression and radiation versus radiation alone for prostate cancer: a randomized trial. JAMA 299, 289–295 (2008).
Jones, C. U. et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 365, 107–118 (2011).
Laverdiere, J. et al. The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2–T3 prostate cancer. J. Urol. 171, 1137–1140 (2004).
Roach, M. 3rd et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J. Clin. Oncol. 26, 585–591 (2008).
Denham, J. W. et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 6, 841–850 (2005).
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002).
Lawton, C. A. et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85–31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 49, 937–946 (2001).
Merrick, G. S., Wallner, K. E. & Butler, W. M. Permanent interstitial brachytherapy for the management of carcinoma of the prostate gland. J. Urol. 169, 1643–1652 (2003).
Zaorsky, N. G. et al. ACR Appropriateness Criteria® External beam radiation therapy treatment planning for clinically localized prostate cancer, part II of II. Adv. Radiat. Oncol. http://dx.doi.org/10.1016/j.adro.2017.03.003 (2017).
Zaorsky, N. G. et al. ACR Appropriateness Criteria® external beam radiation therapy treatment planning for clinically localized prostate cancer, part I of II. Adv. Radiat. Oncol. 2, 62–84 (2017).
Bice, W. S. et al. Recommendations for permanent prostate brachytherapy with 131Cs: a consensus report from the Cesium Advisory Group. Brachytherapy 7, 290–296 (2008).
Polo, A. et al. Review of intraoperative imaging and planning techniques in permanent seed prostate brachytherapy. Radiother. Oncol. 94, 12–23 (2010).
Stone, N. N., Hong, S., Lo, Y. C., Howard, V. & Stock, R. G. Comparison of intraoperative dosimetric implant representation with postimplant dosimetry in patients receiving prostate brachytherapy. Brachytherapy 2, 17–25 (2003).
Zauls, A. J., Ashenafi, M. S., Onicescu, G., Clarke, H. S. & Marshall, D. T. Comparison of intraoperatively built custom linked seeds versus loose seed gun applicator technique using real-time intraoperative planning for permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 81, 1010–1016 (2011).
Prestidge, B. R. et al. A survey of current clinical practice of permanent prostate brachytherapy in the United States. Int. J. Radiat. Oncol. Biol. Phys. 40, 461–465 (1998).
Orio, P. F. 3rd et al. Intraoperative ultrasound-fluoroscopy fusion can enhance prostate brachytherapy quality. Int. J. Radiat. Oncol. Biol. Phys. 69, 302–307 (2007).
Shaikh, T. et al. Is it necessary to perform week three dosimetric analysis in low-dose-rate brachytherapy for prostate cancer when day 0 dosimetry is done? A quality assurance assessment. Brachytherapy 14, 316–321 (2015).
Expert Panel on Radiation, O.-P. et al. American College of Radiology Appropriateness Criteria permanent source brachytherapy for prostate cancer. Brachytherapy 10, 357–362 (2011).
Crook, J., McLean, M., Yeung, I., Williams, T. & Lockwood, G. MRI–CT fusion to assess postbrachytherapy prostate volume and the effects of prolonged edema on dosimetry following transperineal interstitial permanent prostate brachytherapy. Brachytherapy 3, 55–60 (2004).
Tanaka, O. et al. Comparison of MRI-based and CT/MRI fusion-based postimplant dosimetric analysis of prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 66, 597–602 (2006).
Soni, P. D., Berlin, A., Venkatesan, A. M. & McLaughlin, P. W. MRI-guided functional anatomy approach to prostate brachytherapy. Brachytherapy (2016).
Kataria, T. et al. Simple diagrammatic method to delineate male urethra in prostate cancer radiotherapy: an MRI based approach. Br. J. Radiol. 89, 20160348 (2016).
Rylander, S., Buus, S., Pedersen, E. M., Bentzen, L. & Tanderup, K. Dosimetric impact of contouring and needle reconstruction uncertainties in US-, CT- and MRI-based high-dose-rate prostate brachytherapy treatment planning. Radiother. Oncol. (2017).
Buus, S. et al. Learning curve of MRI-based planning for high-dose-rate brachytherapy for prostate cancer. Brachytherapy 15, 426–434 (2016).
Hosni, A. et al. Dosimetric feasibility of ablative dose escalated focal monotherapy with MRI-guided high-dose-rate (HDR) brachytherapy for prostate cancer. Radiother. Oncol. 122, 103–108 (2017).
Venkatesan, A. M. et al. Prostate MRI for brachytherapists: anatomy and technique. Brachytherapy http://dx.doi.org/10.1016/j.brachy.2016.11.009 (2017).
Thaker, N. G., Orio, P. F. & Potters, L. Defining the value of magnetic resonance imaging in prostate brachytherapy using time-driven activity-based costing. Brachytherapy http://dx.doi.org/10.1016/j.brachy.2016.11.013 (2017).
Yu, Y. et al. Permanent prostate seed implant brachytherapy: report of the American Association of Physicists in Medicine Task Group No. 64. Med. Phys. 26, 2054–2076 (1999).
Williamson, J. F. et al. Recommendations of the American Association of Physicists in Medicine on 103Pd interstitial source calibration and dosimetry: implications for dose specification and prescription. Med. Phys. 27, 634–642 (2000).
Crook, J. M., Potters, L., Stock, R. G. & Zelefsky, M. J. Critical organ dosimetry in permanent seed prostate brachytherapy: defining the organs at risk. Brachytherapy 4, 186–194 (2005).
Nath, R. et al. AAPM recommendations on dose prescription and reporting methods for permanent interstitial brachytherapy for prostate cancer: report of Task Group 137. Med. Phys. 36, 5310–5322 (2009).
Gillan, C. et al. Radiation dose to the internal pudendal arteries from permanent-seed prostate brachytherapy as determined by time-of-flight MR angiography. Int. J. Radiat. Oncol. Biol. Phys. 65, 688–693 (2006).
Merrick, G. S. et al. The importance of radiation doses to the penile bulb versus crura in the development of postbrachytherapy erectile dysfunction. Int. J. Radiat. Oncol. Biol. Phys. 54, 1055–1062 (2002).
Buyyounouski, M. K. et al. The radiation doses to erectile tissues defined with magnetic resonance imaging after intensity-modulated radiation therapy or iodine-125 brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 59, 1383–1391 (2004).
Wallner, K. et al. 125I versus 103Pd for low-risk prostate cancer: preliminary PSA outcomes from a prospective randomized multicenter trial. Int. J. Radiat. Oncol. Biol. Phys. 57, 1297–1303 (2003).
Peschel, R. E., Colberg, J. W., Chen, Z., Nath, R. & Wilson, L. D. Iodine 125 versus palladium 103 implants for prostate cancer: clinical outcomes and complications. Cancer J. 10, 170–174 (2004).
Stock, R. G., Stone, N. N., Dahlal, M. & Lo, Y. C. What is the optimal dose for 125I prostate implants? A dose–response analysis of biochemical control, posttreatment prostate biopsies, and long-term urinary symptoms. Brachytherapy 1, 83–89 (2002).
Yoshioka, Y. et al. External-beam radiotherapy for clinically localized prostate cancer in Osaka, Japan, 1995–2006: time trends, outcome, and risk stratification. Strahlenther. Onkol. 185, 446–452 (2009).
Kovacs, G. et al. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother. Oncol. 74, 137–148 (2005).
Pisansky, T. M. et al. High-dose-rate brachytherapy in the curative treatment of patients with localized prostate cancer. Mayo Clin. Proc. 83, 1364–1372 (2008).
Fowler, J., Chappell, R. & Ritter, M. Is alpha/beta for prostate tumors really low? Int. J. Radiat. Oncol. Biol. Phys. 50, 1021–1031 (2001).
Yoshioka, Y. et al. Evaluation of anatomy-based dwell position and inverse optimization in high-dose-rate brachytherapy of prostate cancer: a dosimetric comparison to a conventional cylindrical dwell position, geometric optimization, and dose-point optimization. Radiother. Oncol. 75, 311–317 (2005).
Sumida, I. et al. Optimization of dose distribution for HDR brachytherapy of the prostate using Attraction–Repulsion Model. Int. J. Radiat. Oncol. Biol. Phys. 64, 643–649 (2006).
Van de Werf, E., Lievens, Y., Verstraete, J., Pauwels, K. & Van den Bogaert, W. Time and motion study of radiotherapy delivery: Economic burden of increased quality assurance and IMRT. Radiother. Oncol. 93, 137–140 (2009).
Shah, C. et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy 11, 441–445 (2012).
Parthan, A. et al. CyberKnife for prostate cancer: Is it cost-effective? J. Clin. Oncol. 29 (suppl 7), 87 (2011).
Lievens, Y., van den Bogaert, W. & Kesteloot, K. Activity-based costing: a practical model for cost calculation in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57, 522–535 (2003).
Norlund, A. Costs of radiotherapy. Acta Oncol. 42, 411–415 (2003).
Bauman, G., Rumble, R. B., Chen, J., Loblaw, A. & Warde, P. Intensity-modulated radiotherapy in the treatment of prostate cancer. Clin. Oncol. 24, 461–473 (2012).
Demanes, D. J. et al. High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 81, 1286–1292 (2011).
Barkati, M. et al. High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: a phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 82, 1889–1896 (2012).
Ghadjar, P. et al. Toxicity and early treatment outcomes in low- and intermediate-risk prostate cancer managed by high-dose-rate brachytherapy as a monotherapy. Brachytherapy 8, 45–51 (2009).
Ghilezan, M. et al. High-dose-rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate-risk prostate cancer: preliminary toxicity data. Int. J. Radiat. Oncol. Biol. Phys. 83, 927–932 (2012).
Grills, I. S. et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J. Urol. 171, 1098–1104 (2004).
Hayes, J. et al. Post-treatment PSA kinetics of three prostate cancer treatment regimens involving brachytherapy. Brachytherapy 5, 106 (2006).
Mark, R. J. et al. Interstitial high dose rate (HDR) brachtherapy as monotherapy for early stage prostate cancer: a report of 206 cases. Int. J. Radiat. Oncol. 69, S329–S329 (2007).
Mark, R., Anderson, P. J., Akins, R. S. & Nair, M. High-dose-rate brachytherapy under local anesthesia for early stage prostate cancer: a report of 546 cases. Brachytherapy 10, S93 (2011).
Prada, P. J. et al. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy 11, 105–110 (2012).
Rogers, C. L. et al. High dose brachytherapy as monotherapy for intermediate risk prostate cancer. J. Urol. 187, 109–116 (2012).
Sullivan, L. et al. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother. Oncol. 91, 232–236 (2009).
Yoshioka, Y. et al. High-dose-rate brachytherapy without external beam irradiation for locally advanced prostate cancer. Radiother. Oncol. 80, 62–68 (2006).
Yoshioka, Y. et al. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int. J. Radiat. Oncol. Biol. Phys. 80, 469–475 (2011).
Zamboglou, N. et al. High-dose-rate interstitial brachytherapy as monotherapy for clinically localized prostate cancer: treatment evolution and mature results. Int. J. Radiat. Oncol. Biol. Phys. 85, 672–678 (2012).
Hoskin, P. et al. High-dose-rate brachytherapy with two or three fractions as monotherapy in the treatment of locally advanced prostate cancer. Radiother. Oncol. 112, 63–67 (2014).
Kotecha, R. et al. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy 12, 44–49 (2013).
Phan, T. P., Syed, A. M., Puthawala, A., Sharma, A. & Khan, F. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J. Urol. 177, 123–127 (2007).
Vicini, F. A., Kestin, L. L. & Martinez, A. A. Use of conformal high-dose rate brachytherapy for management of patients with prostate cancer: optimizing dose escalation. Tech. Urol. 6, 135–145 (2000).
Khor, R. et al. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost versus external beam radiation therapy alone for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 85, 679–685 (2013).
Tang, J. I., Williams, S. G., Tai, K. H., Dean, J. & Duchesne, G. M. A prospective dose escalation trial of high-dose-rate brachytherapy boost for prostate cancer: evidence of hypofractionation efficacy? Brachytherapy 5, 256–261 (2006).
Zwahlen, D. R., Andrianopoulos, N., Matheson, B., Duchesne, G. M. & Millar, J. L. High-dose-rate brachytherapy in combination with conformal external beam radiotherapy in the treatment of prostate cancer. Brachytherapy 9, 27–35 (2010).
Ares, C. et al. Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int. J. Radiat. Oncol. Biol. Phys. 75, 656–663 (2009).
Pistis, F. et al. External beam radiotherapy plus high-dose-rate brachytherapy for treatment of locally advanced prostate cancer: the initial experience of the Catalan Institute of Oncology. Brachytherapy 9, 15–22 (2010).
Nohara, T. et al. Clinical results of iridium-192 high dose rate brachytherapy with external beam radiotherapy. Jpn J. Clin. Oncol. 40, 677–683 (2010).
Sato, M. et al. High-dose-rate brachytherapy of a single implant with two fractions combined with external beam radiotherapy for hormone-naive prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 72, 1002–1009 (2008).
Akimoto, T. et al. Rectal bleeding after high-dose-rate brachytherapy combined with hypofractionated external-beam radiotherapy for localized prostate cancer: impact of rectal dose in high-dose-rate brachytherapy on occurrence of grade 2 or worse rectal bleeding. Int. J. Radiat. Oncol. Biol. Phys. 65, 364–370 (2006).
Bachand, F., Martin, A. G., Beaulieu, L., Harel, F. & Vigneault, E. An eight-year experience of HDR brachytherapy boost for localized prostate cancer: biopsy and PSA outcome. Int. J. Radiat. Oncol. Biol. Phys. 73, 679–684 (2009).
Hurwitz, M. D. Technology insight: combined external-beam radiation therapy and brachytherapy in the management of prostate cancer. Nat. Clin. Pract. Oncol. 5, 668–676 (2008).
Aluwini, S. et al. High-dose-rate brachytherapy and external-beam radiotherapy for hormone-naive low- and intermediate-risk prostate cancer: a 7-year experience. Int. J. Radiat. Oncol. Biol. Phys. 83, 1480–1485 (2012).
Deger, S. et al. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur. Urol. 47, 441–448 (2005).
Martin, T. et al. 3D conformal HDR brachytherapy and external beam irradiation combined with temporary androgen deprivation in the treatment of localized prostate cancer. Radiother. Oncol. 71, 35–41 (2004).
Curran, M. J., Healey, G. A., Bihrle, W. 3rd, Goodman, N. & Roth, R. A. Treatment of high-grade low-stage prostate cancer by high-dose-rate brachytherapy. J. Endourol. 14, 351–356 (2000).
Demanes, D. J., Brandt, D., Schour, L. & Hill, D. R. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am. J. Clin. Oncol. 32, 342–347 (2009).
Demanes, D. J., Rodriguez, R. R., Schour, L., Brandt, D. & Altieri, G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 61, 1306–1316 (2005).
Yamada, Y. et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy 5, 157–164 (2006).
Izard, M. A. et al. Six year experience of external beam radiotherapy, brachytherapy boost with a 1 Ci 192Ir source, and neoadjuvant hormonal manipulation for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 66, 38–47 (2006).
Whalley, D. et al. HDR brachytherapy combined with external beam radiation for localised prostate cancer: early experience from the Sydney Cancer Centre. J. Med. Imag. Radiat. Oncol. 56, 220–226 (2012).
Chen, Y. C. et al. High-dose-rate brachytherapy plus external beam radiotherapy for T1 to T3 prostate cancer: an experience in Taiwan. Urology 70, 101–105 (2007).
Luo, H. L., Fang, F. M., Kang, C. H., Chuang, Y. C. & Chiang, P. H. Can high-dose-rate brachytherapy prevent the major genitourinary complication better than external beam radiation alone for patients with previous transurethral resection of prostate? Int. Urol. Nephrol. 45, 113–119 (2013).
Pellizzon, A. C. et al. The relationship between the biochemical control outcomes and the quality of planning of high-dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int. J. Med. Sci. 5, 113–120 (2008).
Agoston, P. et al. Moderate dose escalation with single-fraction high-dose-rate brachytherapy boost for clinically localized intermediate- and high-risk prostate cancer: 5-year outcome of the first 100 consecutively treated patients. Brachytherapy 10, 376–384 (2011).
Galalae, R. M. et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther. Onkol. 180, 582–589 (2004).
Prada, P. J. et al. Long-term biochemical results after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat. Oncol. 7, 31–39 (2012).
Borghede, G. et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother. Oncol. 44, 237–244 (1997).
Kestin, L. L. et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J. Clin. Oncol. 18, 2869–2880 (2000).
Hiratsuka, J. et al. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 59, 684–690 (2004).
Fertil, B. & Malaise, E. P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int. J. Radiat. Oncol. Biol. Phys. 11, 1699–1707 (1985).
Miralbell, R., Roberts, S. A., Zubizarreta, E. & Hendry, J. H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9-2.2) Gy. Int. J. Radiat. Oncol. Biol. Phys. 82, e17–e24 (2012).
Fowler, J. F., Ritter, M. A., Chappell, R. J. & Brenner, D. J. What hypofractionated protocols should be tested for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 56, 1093–1104 (2003).
Brenner, D. J. et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int. J. Radiat. Oncol. Biol. Phys. 52, 6–13 (2002).
Park, C., Papiez, L., Zhang, S., Story, M. & Timmerman, R. D. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 70, 847–852 (2008).
Kwilas, A. R., Donahue, R. N., Bernstein, M. B. & Hodge, J. W. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Frontiers Oncol. 2, 104 (2012).
Meng, M. B. et al. Necroptosis in tumorigenesis, activation of anti-tumor immunity, and cancer therapy. Oncotarget 7, 57391–57413 (2016).
Meng, M. B. et al. Pericytes: a double-edged sword in cancer therapy. Future Oncol. 11, 169–179 (2014).
Wang, H. H. et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 375, 349–359 (2016).
Stock, R. G., Stone, N. N., Tabert, A., Iannuzzi, C. & DeWyngaert, J. K. A dose-response study for I-125 prostate implants. Int. J. Radiat. Oncol. Biol. Phys. 41, 101–108 (1998).
Haynes, B. Can it work? Does it work? Is it worth it? The testing of healthcareinterventions is evolving. BMJ 319, 652–653 (1999).
Kesteloot, K., Lievens, Y. & van der Schueren, E. Improved management of radiotherapy departments through accurate cost data. Radiother. Oncol. 55, 251–262 (2000).
Crook, J. M. et al. Comparison of health-related quality of life 5 years after SPIRIT: Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial. J. Clin. Oncol. 29, 362–368 (2011).
Hoskin, P. et al. High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int. J. Radiat. Oncol. Biol. Phys. 82, 1376–1384 (2012).
Potters, L. et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J. Urol. 173, 1562–1566 (2005).
Stone, N. N. et al. Customized dose prescription for permanent prostate brachytherapy: insights from a multicenter analysis of dosimetry outcomes. Int. J. Radiat. Oncol. Biol. Phys. 69, 1472–1477 (2007).
Zelefsky, M. J. et al. Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int. J. Radiat. Oncol. Biol. Phys. 67, 327–333 (2007).
Grimm, P. et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 109 (Suppl. 1), 22–29 (2012).
Davis, B. J. et al. The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer 85, 2630–2637 (1999).
Sohayda, C., Kupelian, P. A., Levin, H. S. & Klein, E. A. Extent of extracapsular extension in localized prostate cancer. Urology 55, 382–386 (2000).
Teh, B. S., Bastasch, M. D., Mai, W.-Y., Butler, E. B. & Wheeler, T. M. Predictors of extracapsular extension and its radial distance in prostate cancer: implications for prostate IMRT, brachytherapy, and surgery. Cancer J. 9, 454–460 (2003).
Chao, K. K. et al. Clinicopathologic analysis of extracapsular extension in prostate cancer: should the clinical target volume be expanded posterolaterally to account for microscopic extension? Int. J. Radiat. Oncol. Biol. Phys. 65, 999–1007 (2006).
Cosset, J. M. et al. Selecting patients for exclusive permanent implant prostate brachytherapy: the experience of the Paris Institut Curie/Cochin Hospital/Necker Hospital group on 809 patients. Int. J. Radiat. Oncol. Biol. Phys. 71, 1042–1048 (2008).
Prestidge, B. R. et al. Initial Report of NRG Oncology/RTOG 0232: a phase III study comparing combined external beam radiation and transperineal interstitial permanent brachytherapy with brachytherapy alone for selected patients with intermediate risk prostatic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 96, S4 (2016).
Frank, S. J. et al. Interstitial implant alone or in combination with external beam radiation therapy for intermediate-risk prostate cancer: a survey of practice patterns in the United States. Brachytherapy 6, 2–8 (2007).
Lee, W. R. et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer 109, 1506–1512 (2007).
Hurwitz, M. D. et al. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer 117, 5579–5588 (2011).
Serrano, N. et al. Comparative study of late rectal toxicity in prostate cancer patients treated with low-dose-rate brachytherapy: with or without supplemental external beam radiotherapy. Brachytherapy 15, 435–441 (2016).
Wang, L. S. et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer 121, 3010–3017 (2015).
Zaorsky, N. G. et al. Prostate cancer patients with unmanaged diabetes or receiving insulin experience inferior outcomes and toxicities after treatment with radiation therapy. Clin. Genitourin. Cancer 15, 326–335 (2017).
Horwitz, E. M. et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J. Clin. Oncol. 26, 2497–2504 (2008).
Bolla, M. et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 11, 1066–1073.
Granfors, T., Modig, H., Damber, J. E. & Tomic, R. Long-term followup of a randomized study of locally advanced prostate cancer treated with combined orchiectomy and external radiotherapy versus radiotherapy alone. J. Urol. 176, 544–547 (2006).
Merrick, G. S. et al. Androgen deprivation therapy does not impact cause-specific or overall survival in high-risk prostate cancer managed with brachytherapy and supplemental external beam. Int. J. Radiat. Oncol. Biol. Phys. 68, 34–40 (2007).
Stone, N. N. et al. Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason score 7–10 prostate cancer treated with permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 73, 341–346 (2009).
Hauswald, H. et al. High-dose-rate monotherapy for localized prostate cancer: 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 94, 667–674 (2016).
Yoshioka, Y. Current status and perspectives of brachytherapy for prostate cancer. Int. J. Clin. Oncol. 14, 31–36 (2009).
Zaorsky, N. G. et al. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother. Oncol. 115, 295–300 (2015).
Nomiya, T. et al. Management of high-risk prostate cancer: radiation therapy and hormonal therapy. Cancer Treat. Rev. 39, 872–878 (2013).
Zaorsky, N. G. et al. Impact of radiation therapy dose escalation on prostate cancer outcomes and toxicities. Am. J. Clin. Oncol. https://doi.org/10.1097/COC.0000000000000285 (2016).
Allen, G. W., Howard, A. R., Jarrard, D. F. & Ritter, M. A. Management of prostate cancer recurrences after radiation therapy–brachytherapy as a salvage option. Cancer 110, 1405–1416 (2007).
Tetreault-Laflamme, A. & Crook, J. Options for salvage of radiation failures for prostate cancer. Semin. Radiat. Oncol. 27, 67–78 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00450411 (2016).
Tharp, M. et al. Prostate high-dose-rate brachytherapy as salvage treatment of local failure after previous external or permanent seed irradiation for prostate cancer. Brachytherapy 7, 231–236 (2008).
Lee, B. et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco experience. Int. J. Radiat. Oncol. Biol. Phys. 67, 1106–1112 (2007).
Nguyen, P. L. et al. Magnetic resonance image-guided salvage brachytherapy after radiation in select men who initially presented with favorable-risk prostate cancer: a prospective phase 2 study. Cancer 110, 1485–1492 (2007).
Nguyen, P. L., D'Amico, A. V., Lee, A. K. & Suh, W. W. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer 110, 1417–1428 (2007).
Nguyen, P. L. et al. Patient-reported quality of life after salvage brachytherapy for radio-recurrent prostate cancer: a prospective phase II study. Brachytherapy 8, 345–352 (2009).
Nguyen, P. L. et al. High-dose-rate brachytherapy for prostate cancer in a previously radiated patient with polyethylene glycol hydrogel spacing to reduce rectal dose: case report and review of the literature. Brachytherapy 12, 77–83 (2013).
Tree, A. C. et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 14, e28–e37 (2013).
Yao, H. H., Hong, M., Corcoran, N. M., Siva, S. & Foroudi, F. Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia Pac. J. Clin. Oncol. 10, 308–321 (2014).
Zaorsky, N. G., Raj, G. V., Trabulsi, E. J., Lin, J. & Den, R. B. The dilemma of a rising prostate-specific antigen level after local therapy: what are our options? Semin. Oncol. 40, 322–336 (2013).
Valerio, M. et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur. Urol. 66, 732–751 (2014).
Chen, R. C. et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J. Natl Cancer Inst. 106, dju132 (2014).
Wilt, T. J. et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann. Internal Med. 148, 435–448 (2008).
Efficace, F. et al. Patient-reported outcomes in randomised controlled trials of prostate cancer: methodological quality and impact on clinical decision making. Eur. Urol. 66, 416–427 (2014).
Punnen, S., Cowan, J. E., Chan, J. M., Carroll, P. R. & Cooperberg, M. R. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur. Urol. 68, 600–608 (2015).
Chen, R. C., Clark, J. A. & Talcott, J. A. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J. Clin. Oncol. 27, 3916–3922 (2009).
Schmidt, S. et al. Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual. Life Res. 23, 2169–2181 (2014).
Lloyd, A. J., Kerr, C., Penton, J. & Knerer, G. Health-related quality of life and health utilities in metastatic castrate-resistant prostate cancer: a survey capturing experiences from a diverse sample of UK patients. Value Health 18, 1152–1157 (2015).
Barry, M. J. et al. The American Urological Association symptom index for benign prostatic hyperplasia. J. Urol. 148, 1549–1557; discussion 1564 (1992).
Rosen, R. C. et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49, 822–830 (1997).
NIH Consensus Development Panel on Impotence. Impotence. JAMA 270, 83–90 (1993).
Johnson, M. E. et al. Patient reported outcomes among treatment modalities for prostate cancer. Can. J. Urol. 23, 8535–8545 (2016).
Reis, L. O., Sanches, B. C., Zani, E. L., Castilho, L. N. & Monti, C. R. PSA-nadir at 1 year as a sound contemporary prognostic factor for low-dose-rate iodine-125 seeds brachytherapy. World J. Urol. 32, 753–759 (2014).
Hackett, C. et al. Distinguishing prostate-specific antigen bounces from biochemical failure after low-dose-rate prostate brachytherapy. J. Contemp. Brachyther. 6, 247–253 (2014).
Tanaka, N. et al. Minimal percentage of dose received by 90% of the urethra (%UD90) is the most significant predictor of PSA bounce in patients who underwent low-dose-rate brachytherapy (LDR-brachytherapy) for prostate cancer. BMC Urol. 12, 28 (2012).
Reed, D., Wallner, K., Merrick, G., Buskirk, S. & True, L. Clinical correlates to PSA spikes and positive repeat biopsies after prostate brachytherapy. Urology 62, 683–688 (2003).
McGrath, S. D. et al. PSA bounce after prostate brachytherapy with or without neoadjuvant androgen deprivation. Brachytherapy 9, 137–144 (2010).
Naghavi, A. O. et al. Clinical implications of a prostate-specific antigen bounce after radiation therapy for prostate cancer. Int. J. Clin. Oncol. 20, 598–604 (2015).
Fuller, D. B., Naitoh, J. & Mardirossian, G. Virtual HDR CyberKnife SBRT for localized prostatic carcinoma: 5-year disease-free survival and toxicity observations. Front. Oncol. 4, 321 (2014).
Pinkawa, M. et al. Prostate-specific antigen kinetics following external-beam radiotherapy and temporary (Ir-192) or permanent (I-125) brachytherapy for prostate cancer. Radiother. Oncol. 96, 25–29 (2010).
Patel, N. et al. Prostate-specific antigen bounce after high-dose-rate prostate brachytherapy and hypofractionated external beam radiotherapy. Brachytherapy 13, 450–455 (2014).
Mehta, N. H., Kamrava, M., Wang, P. C., Steinberg, M. & Demanes, J. Prostate-specific antigen bounce after high-dose-rate monotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 86, 729–733 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02628041 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02346253 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02258087 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02560181 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02322931 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02225925 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02632669 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02290366 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00913939 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01936883 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01909388 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02790216 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02652000 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02597894 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02805894 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01437085 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02623933 (2017).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, N. G. Z., B. J. D., P. L. N, T. N. S, P. H., Y. Y., and G. C. M made a substantial contribution to discussion of content. N. G. Z. and E. M. H. wrote the manuscript. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
PowerPoint slides
Glossary
- Remote afterloading system
-
(RALS). Integral to HDR-BT, a RALS automatically deploys and retracts a single small radioactive source along the implant needle at specific positions delivering ≥12 Gy/h. The RALS enables a physician to control the position where the HDR source stops for a predetermined time period (the dwell position and dwell time).
- Hypofractionated radiation therapy
-
A type of EBRT that is delivered as a single 2.1–3.5 Gy fraction lasting 15 min per day, 5 days per week, for about 4 weeks.
- Stereotactic body radiation therapy
-
(SBRT). A type of EBRT delivered as a single fraction ∼ 6.0–9.0 Gy lasting up to 45 min per day, for a total of about five treatments over about 2 weeks.
- Gross tumour volume
-
(GTV). The demonstrable extent and location of the malignant growth; it consists of the primary tumour, which for prostate cancer has historically been defined as the entire gland as well as any visualized extension into surrounding normal tissues, the regional lymph nodes, or distant metastases based on clinical data.
- Clinical target volume
-
(CTV). This volume encompasses the GTV as well as areas at risk for subclinical cancer involvement. The CTV can include a margin around the prostate GTV and adjacent regions at risk of having subclinical disease.
- Planning target volume
-
(PTV). This volume encompasses the CTV plus an additional margin to account for patient movement, setup error, and organ movement
- Dwell positions
-
The positions where a 192Ir source is located during HDR-BT. A combination of dwell positions in different needles enables the delivery of a predetermined dose to the CTV.
- Dwell times
-
The times that the 192Ir source spends in a predetermined dwell position during HDR-BT. A longer dwell time in a position translates to a greater dose deposited in the volume around the position.
- Equivalent dose in 2 Gy fractions
-
(EQD2). The “2 Gy-per-fraction equivalent dose.” EQD2 = n*d*((d + α/β)/(2 + α/β)). The EQD2 uses a mathematical conversion of fractions and dose per fraction, similar to the BED. In this formula, n is the number of radiation fractions and d is the dose size per fraction.
- D90
-
In prostate cancer brachytherapy, this is the minimum dose in the hottest 90% of a volume, in Gy. The prostate D90% should be >100%. This constraint ensures the prostate volume receives adequate dose.
- V100
-
In prostate cancer brachytherapy, this is the percentage of a structure receiving 100% of the dose. For example, the V100 for the prostate should be >90%, meaning that 100% of the prostate CTV should receive more than 90% of the prescribed dose.
- V150
-
In prostate cancer brachytherapy, this is the percentage of a structure receiving 150% of the dose. The V150 for the prostate CTV should be <50–60%, meaning that <50–60% of the CTV should receive >150% of the prescribed dose.
- UV150
-
In prostate cancer brachytherapy, this is the volume of the urethra receiving 150% of the prescribe dose. UV150 of the urethra should be 0%, meaning that 0% of the volume should receive 150% of the prescribed dose.
- UV5
-
In prostate cancer brachytherapy, this is the average dose to 5% of the urethral volume receiving the highest dose. The UV5 should receive <150% of the dose.
- UV30
-
In prostate cancer brachytherapy, this is the average dose to 30% of the urethral volume receiving the highest dose. The UV30 should be <125% of the dose.
- D10
-
The average dose to 10% of a volume, in Gy. The urethra D10 should be <150% of the prescribed dose. This constraint limits the dose to the urethra.
- D30
-
The average dose to 30% of a volume, in Gy. The urethra D30 should be <130% of the prescribed dose. This constraint limits the dose to the urethra.
- RV100
-
In prostate cancer brachytherapy, this is the volume of the rectum receiving 100% of the dose, and should be <1 cc.
- D2cc
-
The average dose to 2 cc of a volume.
- D0.1cc or Dmax
-
The average dose to the hottest point of a volume. The term “D0.1cc” is sometimes used because this approximates the maximum dose to the smallest volume that can be calculated on a computer.
- Hotspot
-
A colloquialism used to describe volume outside the PTV which receives a dose >100% of the specified PTV dose.
- α/β ratio
-
The α/β ratio describes the shape of the cell survival curve and the gradient of the two components of cell kill, α and β. The α/β ratio is used to describe the dose response of radiation on different tissues. Prostate cancer cells have a relatively low α/β ratio of 1.5, implying that those cells are more sensitive to doses delivered in larger fraction size. In the radiobiological linear quadratic equation, it is the dose at which cell killing due to the linear and quadratic components are equal.
- Biologically equivalent dose
-
(BED). A more conceptually useful measure of biological damage to cells than physical dose. It takes into account the α/β ratio, number of radiation fractions, and fraction size. BED = (nd[1 + d/(α/β)]). In this formula, n is the number of radiation fractions and d is the dose size per fraction.
- Intensity-modulated radiation therapy
-
(IMRT). An advanced form of high-precision radiation that conforms the treatment volume to the shape of the tumour. The dose distribution created by IMRT is characterized by a concavity or invagination of the edge of the higher doses away from the rectum, rather than a straight edge through the rectum as seen with 3D conformal radiation therapy.
- Multi-leaf collimator
-
(MLC). A device made up of individual leaves of a high atomic numbered material that can move independently in and out of the path of an X-ray beam to contour its shape to a tumour.
- Phoenix definition
-
Used for measuring biochemical failure after radiotherapy for prostate cancer, defined as the PSA nadir value plus 2 ng/ml.
Rights and permissions
About this article
Cite this article
Zaorsky, N., Davis, B., Nguyen, P. et al. The evolution of brachytherapy for prostate cancer. Nat Rev Urol 14, 415–439 (2017). https://doi.org/10.1038/nrurol.2017.76
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2017.76
This article is cited by
-
Review of brachytherapy clinical trials: a cross-sectional analysis of ClinicalTrials.gov
Radiation Oncology (2024)
-
Dosimetry in Lu-177-DOTATATE peptide receptor radionuclide therapy: a systematic review
Clinical and Translational Imaging (2023)
-
Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer
Nature Reviews Clinical Oncology (2022)
-
Network models of prostate cancer immune microenvironments identify ROMO1 as heterogeneity and prognostic marker
Scientific Reports (2022)
-
Using MCNP5 to evaluate radiation doses in prostate brachytherapy with cesium-131
Pramana (2022)