Key Points
-
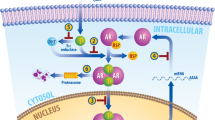
Endothelial transcription factor GATA-2 (GATA2) is a pioneer, master-regulator, transcription factor that binds DNA regions of closed chromatin, causing opening and facilitating subsequent hierarchical binding of other regulators that activate transcription
-
GATA2 is crucial for the development of the genitourinary system and might be a lineage marker of mouse and human prostate tissue
-
GATA2 drives androgen-responsive gene expression and contributes to prostate cancer metastasis by increasing tumour cell motility and invasiveness in early stages of the disease, through its pioneer transcription factor function
-
GATA2 is important in prostate cancer progression to an androgen-refractory state and regulates an androgen-independent signalling network in late stages of the disease
-
Preclinical experimental data have demonstrated the utility of inhibiting GATA2 through targeting its upstream regulators, post-translational modifications, and downstream effectors
-
Integrating GATA2 inhibition into the therapeutic landscape of prostate cancer will require the development of precise predictive assays and identification of the most effective therapeutic combination
Abstract
Advanced prostate cancer is a classic example of the intractability and consequent lethality that characterizes metastatic carcinomas. Novel treatments have improved the survival of men with prostate cancer; however, advanced prostate cancer invariably becomes resistant to these therapies and ultimately progresses to a lethal metastatic stage. Consequently, detailed knowledge of the molecular mechanisms that control prostate cancer cell survival and progression towards this lethal stage of disease will benefit the development of new therapeutics. The transcription factor endothelial transcription factor GATA-2 (GATA2) has been reported to have a key role in driving prostate cancer aggressiveness. In addition to being a pioneer transcription factor that increases androgen receptor (AR) binding and activity, GATA2 regulates a core subset of clinically relevant genes in an AR-independent manner. Functionally, GATA2 overexpression in prostate cancer increases cellular motility and invasiveness, proliferation, tumorigenicity, and resistance to standard therapies. Thus, GATA2 has a multifaceted function in prostate cancer aggressiveness and is a highly attractive target in the development of novel treatments against lethal prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
25 November 2016
In the original version of this article the acknowledgements section was omitted. This has been corrected in the print and online versions of the manuscript.
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Pound, C. R. et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999).
Klotz, L. et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. 33, 272–277 (2015).
Seidenfeld, J. et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann. Intern. Med. 132, 566–577 (2000).
Hellerstedt, B. A. & Pienta, K. J. The current state of hormonal therapy for prostate cancer. CA Cancer J. Clin. 52, 154–179 (2002).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Huggins, C., Stevens, R. E. & Hodges, C. V. Studies on prostate cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 43, 209–223 (1941).
Knudsen, K. E. & Penning, T. M. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 21, 315–324 (2010).
Zong, Y. & Goldstein, A. S. Adaptation or selection — mechanisms of castration-resistant prostate cancer. Nat. Rev. Urol. 10, 90–98 (2013).
Armstrong, C. M. & Gao, A. C. Drug resistance in castration resistant prostate cancer: resistance mechanisms and emerging treatment strategies. Am. J. Clin. Exp. Urol. 3, 64–76 (2015).
Bresnick, E. H., Lee, H. Y., Fujiwara, T., Johnson, K. D. & Keles, S. GATA switches as developmental drivers. J. Biol. Chem. 285, 31087–31093 (2010).
Burch, J. B. Regulation of GATA gene expression during vertebrate development. Semin. Cell Dev. Biol. 16, 71–81 (2005).
Vicente, C., Conchillo, A., Garcia-Sanchez, M. A. & Odero, M. D. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol. Hematol. 82, 1–17 (2012).
Zheng, R. & Blobel, G. A. GATA transcription factors and cancer. Genes Cancer 1, 1178–1188 (2010).
Evans, T., Reitman, M. & Felsenfeld, G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc. Natl Acad. Sci. USA 85, 5976–5980 (1988).
Weiss, M. J. & Orkin, S. H. GATA transcription factors: key regulators of hematopoiesis. Exp. Hematol. 23, 99–107 (1995).
Nemer, G. & Nemer, M. Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev. Biol. 254, 131–148 (2003).
Kaplan, T. et al. Quantitative models of the mechanisms that control genome-wide patterns of transcription factor binding during early Drosophila development. PLoS Genet. 7, e1001290 (2011).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Iwafuchi-Doi, M. & Zaret, K. S. Pioneer transcription factors in cell reprogramming. Genes Dev. 28, 2679–2692 (2014).
Gualdi, R. et al. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10, 1670–1682 (1996).
Bossard, P. & Zaret, K. S. GATA transcription factors as potentiators of gut endoderm differentiation. Development 125, 4909–4917 (1998).
Tsai, F. Y. et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–226 (1994).
Tsai, F. Y. & Orkin, S. H. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89, 3636–3643 (1997).
Kumano, K. et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18, 699–711 (2003).
Robert-Moreno, A., Espinosa, L., de la Pompa, J. L. & Bigas, A. RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132, 1117–1126 (2005).
Fujiwara, Y., Browne, C. P., Cunniff, K., Goff, S. C. & Orkin, S. H. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA 93, 12355–12358 (1996).
Orlic, D., Anderson, S., Biesecker, L. G., Sorrentino, B. P. & Bodine, D. M. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, 45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc. Natl Acad. Sci. USA 92, 4601–4605 (1995).
Minegishi, N. et al. Alternative promoters regulate transcription of the mouse GATA-2 gene. J. Biol. Chem. 273, 3625–3634 (1998).
Ling, K. W. et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200, 871–882 (2004).
Lugus, J. J. et al. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development 134, 393–405 (2007).
Xiong, J. W. Molecular and developmental biology of the hemangioblast. Dev. Dyn. 237, 1218–1231 (2008).
Nishikawa, S. I. A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Curr. Opin. Cell Biol. 13, 673–678 (2001).
Gumina, R. J., Kirschbaum, N. E., Piotrowski, K. & Newman, P. J. Characterization of the human platelet/endothelial cell adhesion molecule-1 promoter: identification of a GATA-2 binding element required for optimal transcriptional activity. Blood 89, 1260–1269 (1997).
Lee, M. E., Temizer, D. H., Clifford, J. A. & Quertermous, T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J. Biol. Chem. 266, 16188–16192 (1991).
Kappel, A. et al. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of Flk-1 expression during murine vascular development. Blood 96, 3078–3085 (2000).
Coma, S. et al. GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of neuropilin-2. Angiogenesis 16, 939–952 (2013).
Zhou, Y. et al. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 17, 6689–6700 (1998).
Khandekar, M., Suzuki, N., Lewton, J., Yamamoto, M. & Engel, J. D. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developing urogenital system. Mol. Cell. Biol. 24, 10263–10276 (2004).
Perez-Stable, C. M., Pozas, A. & Roos, B. A. A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol. Cell. Endocrinol. 167, 43–53 (2000).
Spinner, M. A. et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123, 809–821 (2014).
Mansour, S. et al. Emberger syndrome-primary lymphedema with myelodysplasia: report of seven new cases. Am. J. Med. Genet. A 152A, 2287–2296 (2010).
Ostergaard, P. et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 43, 929–931 (2011).
Hsu, A. P. et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 118, 2653–2655 (2011).
Dickinson, R. E. et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 118, 2656–2658 (2011).
Wang, Q. et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27, 380–392 (2007).
Wu, D. et al. Three-tiered role of the pioneer factor GATA2 in promoting androgen-dependent gene expression in prostate cancer. Nucleic Acids Res. 42, 3607–3622 (2014).
Vidal, S. J. et al. A targetable GATA2–IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell 27, 223–239 (2015).
Watahiki, A. et al. MicroRNAs associated with metastatic prostate cancer. PLoS ONE 6, e24950 (2011).
Chiang, Y. T. et al. GATA2 as a potential metastasis-driving gene in prostate cancer. Oncotarget 5, 451–461 (2014).
He, B. et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc. Natl Acad. Sci. USA 111, 18261–18266 (2014).
Bohm, M., Locke, W. J., Sutherland, R. L., Kench, J. G. & Henshall, S. M. A role for GATA-2 in transition to an aggressive phenotype in prostate cancer through modulation of key androgen-regulated genes. Oncogene 28, 3847–3856 (2009).
Cleutjens, K. B. et al. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol. Endocrinol. 11, 148–161 (1997).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Henshall, S. M. et al. Zinc-alpha2-glycoprotein expression as a predictor of metastatic prostate cancer following radical prostatectomy. J. Natl Cancer Inst. 98, 1420–1424 (2006).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 (2009).
Jin, Q. et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 (2011).
Zhao, J. C. et al. FOXA1 acts upstream of GATA2 and AR in hormonal regulation of gene expression. Oncogene 35, 4335–4344 (2016).
Karantanos, T., Corn, P. G. & Thompson, T. C. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32, 5501–5511 (2013).
Harris, W. P., Mostaghel, E. A., Nelson, P. S. & Montgomery, B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 6, 76–85 (2009).
Pienta, K. J. & Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 12, 1665–1671 (2006).
Cookson, M. S. et al. Castration-resistant prostate cancer: AUA guideline. J. Urol. 190, 429–438 (2013).
Debes, J. D. & Tindall, D. J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 351, 1488–1490 (2004).
Dutt, S. S. & Gao, A. C. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 5, 1403–1413 (2009).
Hendriksen, P. J. et al. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 66, 5012–5020 (2006).
Cai, C. et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Rep. 9, 1618–1627 (2014).
Chen, Z. et al. Phospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growth. EMBO J. 30, 2405–2419 (2011).
Bishr, M. & Saad, F. Overview of the latest treatments for castration-resistant prostate cancer. Nat. Rev. Urol. 10, 522–528 (2013).
Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 (2011).
Kumar, M. S. et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 149, 642–655 (2012).
Hoelder, S., Clarke, P. A. & Workman, P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol. Oncol. 6, 155–176 (2012).
Lazo, J. S. & Sharlow, E. R. Drugging undruggable molecular cancer targets. Annu. Rev. Pharmacol. Toxicol. 56, 23–40 (2016).
Zeuner, A. et al. The Notch2–Jagged1 interaction mediates stem cell factor signaling in erythropoiesis. Cell Death Differ. 18, 371–380 (2011).
Catelain, C. et al. The Notch Delta-4 ligand helps to maintain the quiescence and the short-term reconstitutive potential of haematopoietic progenitor cells through activation of a key gene network. Stem Cell Res. 13, 431–441 (2014).
Guiu, J. et al. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J. Exp. Med. 210, 71–84 (2013).
de Pooter, R. F. et al. Notch signaling requires GATA-2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J. Immunol. 176, 5267–5275 (2006).
Kumano, K. et al. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood 98, 3283–3289 (2001).
Robert-Moreno, A. et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 27, 1886–1895 (2008).
Flores, A. N., McDermott, N., Meunier, A. & Marignol, L. NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat. Rev. Urol. 11, 499–507 (2014).
Su, Q. & Xin, L. Notch signaling in prostate cancer: refining a therapeutic opportunity. Histol. Histopathol. 31, 149–157 (2016).
Marignol, L., Rivera-Figueroa, K., Lynch, T. & Hollywood, D. Hypoxia, notch signalling, and prostate cancer. Nat. Rev. Urol. 10, 405–413 (2013).
Domingo-Domenech, J. et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 22, 373–388 (2012).
Bertrand, F. E., McCubrey, J. A., Angus, C. W., Nutter, J. M. & Sigounas, G. NOTCH and PTEN in prostate cancer. Adv. Biol. Regul. 56, 51–65 (2014).
Yuan, X. et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 369, 20–27 (2015).
Espinoza, I. & Miele, L. Notch inhibitors for cancer treatment. Pharmacol. Ther. 139, 95–110 (2013).
Taoudi, S. et al. ERG dependence distinguishes developmental control of hematopoietic stem cell maintenance from hematopoietic specification. Genes Dev. 25, 251–262 (2011).
Lulli, V. et al. Overexpression of Ets-1 in human hematopoietic progenitor cells blocks erythroid and promotes megakaryocytic differentiation. Cell Death Differ. 13, 1064–1074 (2006).
Craven, S. E. et al. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development 131, 1165–1173 (2004).
Suh, J. M. et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 3, 25–34 (2006).
Treier, M. et al. Hedgehog signaling is required for pituitary gland development. Development 128, 377–386 (2001).
Dalgin, G. et al. GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Dev. Biol. 310, 454–469 (2007).
Goldman, O. et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells 27, 1750–1759 (2009).
Maguer-Satta, V. et al. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFβ family. Exp. Cell Res. 282, 110–120 (2003).
Adamo, P. & Ladomery, M. R. The oncogene ERG: a key factor in prostate cancer. Oncogene 35, 403–414 (2016).
Gonnissen, A., Isebaert, S. & Haustermans, K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int. J. Mol. Sci. 14, 13979–14007 (2013).
Tu, W. H. et al. The loss of TGF-β signaling promotes prostate cancer metastasis. Neoplasia 5, 267–277 (2003).
Hayakawa, F. et al. Functional regulation of GATA-2 by acetylation. J. Leukoc. Biol. 75, 529–540 (2004).
Ozawa, Y. et al. Histone deacetylase 3 associates with and represses the transcription factor GATA-2. Blood 98, 2116–2123 (2001).
Heemers, H. V., Debes, J. D. & Tindall, D. J. The role of the transcriptional coactivator p300 in prostate cancer progression. Adv. Exp. Med. Biol. 617, 535–540 (2008).
Heemers, H. V. et al. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res. 67, 3422–3430 (2007).
Shah, S., Prasad, S. & Knudsen, K. E. Targeting pioneering factor and hormone receptor cooperative pathways to suppress tumor progression. Cancer Res. 72, 1248–1259 (2012).
Ide, H. et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 70, 1127–1133 (2010).
Thomas, R., Williams, M., Sharma, H., Chaudry, A. & Bellamy, P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer — the U.K. NCRN Pomi-T study. Prostate Cancer Prostat. Dis. 17, 180–186 (2014).
Paller, C. J. et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: safety, tolerability, and dose determination. Prostate 75, 1518–1525 (2015).
Mahammedi, H. et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology 90, 69–78 (2016).
Deguchi, A. Curcumin targets in inflammation and cancer. Endocr. Metab. Immune Disord. Drug Targets 15, 88–96 (2015).
Gupta, S. C. et al. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 559, 91–99 (2014).
Vallianou, N. G., Evangelopoulos, A., Schizas, N. & Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 35, 645–651 (2015).
Imagawa, S. et al. A GATA-specific inhibitor (K-7174) rescues anemia induced by IL-1β, TNF-α, or L-NMMA. FASEB J. 17, 1742–1744 (2003).
Kikuchi, J. et al. Homopiperazine derivatives as a novel class of proteasome inhibitors with a unique mode of proteasome binding. PLoS ONE 8, e60649 (2013).
Minegishi, N., Suzuki, N., Kawatani, Y., Shimizu, R. & Yamamoto, M. Rapid turnover of GATA-2 via ubiquitin-proteasome protein degradation pathway. Genes Cells 10, 693–704 (2005).
Ahn, E. E. et al. SON protein regulates GATA-2 through transcriptional control of the microRNA 23a∼27a∼24–22 cluster. J. Biol. Chem. 288, 5381–5388 (2013).
Towatari, M. et al. Regulation of GATA-2 phosphorylation by mitogen-activated protein kinase and interleukin-3. J. Biol. Chem. 270, 4101–4107 (1995).
Menghini, R. et al. Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue-related inflammation: a novel pathway linking obesity to atherosclerosis. Circulation 111, 1946–1953 (2005).
Katsumura, K. R., Yang, C., Boyer, M. E., Li, L. & Bresnick, E. H. Molecular basis of crosstalk between oncogenic Ras and the master regulator of hematopoiesis GATA-2. EMBO Rep. 15, 938–947 (2014).
Baumgartner, C. & Baccarini, M. p38 links RAS to GATA2. EMBO Rep. 15, 912–913 (2014).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Cellot, S. et al. RNAi screen identifies Jarid1b as a major regulator of mouse HSC activity. Blood 122, 1545–1555 (2013).
Celton, M. et al. Epigenetic regulation of GATA2 and its impact on normal karyotype acute myeloid leukemia. Leukemia 28, 1617–1626 (2014).
Dmitriev, A. A. et al. Identification of novel epigenetic markers of prostate cancer by NotI-microarray analysis. Dis. Markers 2015, 241301 (2015).
Estey, E. H. Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia. Leukemia 27, 1803–1812 (2013).
Moriguchi, T. & Yamamoto, M. A regulatory network governing Gata1 and Gata2 gene transcription orchestrates erythroid lineage differentiation. Int. J. Hematol. 100, 417–424 (2014).
Bresnick, E. H., Katsumura, K. R., Lee, H. Y., Johnson, K. D. & Perkins, A. S. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 40, 5819–5831 (2012).
Livingstone, C. IGF2 and cancer. Endocr. Relat. Cancer 20, R321–R339 (2013).
Mulvihill, M. J. et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 1, 1153–1171 (2009).
Acknowledgements
V.R.-B. receives funding from the U.S. Department of Health & Human Services, NIH, National Cancer Institute grant number 1 K22 CA207458-01 and J.D.-D. receives funding from U.S. Department of Health & Human Services, NIH, National Cancer Institute grant number 1 R01 CA207311-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rodriguez-Bravo, V., Carceles-Cordon, M., Hoshida, Y. et al. The role of GATA2 in lethal prostate cancer aggressiveness. Nat Rev Urol 14, 38–48 (2017). https://doi.org/10.1038/nrurol.2016.225
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.225
This article is cited by
-
The androgen receptor interacts with GATA3 to transcriptionally regulate a luminal epithelial cell phenotype in breast cancer
Genome Biology (2024)
-
Integrative analysis of the ST6GALNAC family identifies GATA2-upregulated ST6GALNAC5 as an adverse prognostic biomarker promoting prostate cancer cell invasion
Cancer Cell International (2023)
-
A GATA2-CDC6 axis modulates androgen receptor blockade-induced senescence in prostate cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
GATA2 co-opts TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to modulate prostate cancer progression
Journal of Experimental & Clinical Cancer Research (2023)
-
Silencing HOXC13 exerts anti-prostate cancer effects by inducing DNA damage and activating cGAS/STING/IRF3 pathway
Journal of Translational Medicine (2023)