Key Points
-
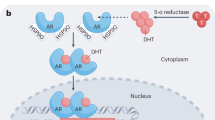
Prostate cancers are capable of intratumoural androgen biosynthesis, which is a potential mechanism of castration resistance
-
Three possible competing androgen biosynthesis pathways exist, all of which might enable androgen biosynthesis in the presence of androgen-deprivation therapy
-
Currently, investigations of androgen biosynthesis rely heavily on preclinical models, which generally do not accurately reflect human disease in this setting
-
Identifying the dominant androgen biosynthetic pathway in each patient could have implications for treatment-related decisions
Abstract
The accumulation of high concentrations of signalling androgens within prostate tumours that progress despite use of androgen-deprivation therapy is a clinically important mechanism of the development of castration-resistant prostate cancer. In the past 5 years, data from a number of studies have increased our understanding of the enzymes and substrates involved in intratumoural androgen biosynthesis, and have implicated three competing pathways, which are likely to account for these observations. These pathways ('canonical', 'backdoor' and '5α-dione'), which can all ultimately generate the potent signalling androgen, dihydrotestosterone, involve many of the same enzymes, but differ in terms of substrate preference, reaction sequence and the organs and tissues in which they occur. For this reason, the relative importance of each pathway to the development and progression of prostate cancer remains controversial. In this Review, we describe the current understanding of androgen synthesis and the evidence for its role in castration resistance, and examine the evidence supporting and or rebutting the relevance of each pathway to patients with prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
James, N. D. et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur. Urol. 67, 1028–1038 (2015).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Scher, H. I. & Sawyers, C. L. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 23, 8253–8261 (2005).
Mostaghel, E. A. et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 67, 5033–5041 (2007).
Liu, J., Geller, J., Albert, J. & Kirshner, M. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J. Clin. Endocrinol. Metab. 61, 129–133 (1985).
Geller, J. et al. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin. Endocrinol. 26, 155–161 (1987).
Labrie, F. et al. Combination therapy with flutamide and castration (LHRH agonist or orchiectomy) in advanced prostate cancer: a marked improvement in response and survival. J. Steroid Biochem. 23, 833–841 (1985).
Gregory, C. W. et al. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61, 2892–2898 (2001).
Mohler, J. L. et al. The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 10, 440–448 (2004).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Labrie, F. Blockade of testicular and adrenal androgens in prostate cancer treatment. Nat. Rev. Urol. 8, 73–85 (2011).
Midzak, A. S., Chen, H., Papadopoulos, V. & Zirkin, B. R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 299, 23–31 (2009).
Sharifi, N. & Auchus, R. J. Steroid biosynthesis and prostate cancer. Steroids 77, 719–726 (2012).
Andersson, S. et al. Molecular genetics and pathophysiology of 17 beta-hydroxysteroid dehydrogenase 3 deficiency. J. Clin. Endocrinol. Metab. 81, 130–136 (1996).
Labrie, F. et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr. Rev. 26, 361–379 (2005).
Labrie, F., Dupont, A. & Belanger, A. Complete androgen blockade for the treatment of prostate cancer. Important Adv. Oncol. 193–217 (1985).
Labrie, F. et al. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J. Steroid Biochem. Mol. Biol. 113, 52–56 (2009).
Vermeulen, A., Schelfhout, W. & De Sy, W. Plasma androgen levels after subcapsular orchiectomy or estrogen treatment for prostatic carcinoma. Prostate 3, 115–121 (1982).
Belanger, A., Dupont, A. & Labrie, F. Inhibition of basal and adrenocorticotropin-stimulated plasma levels of adrenal androgens after treatment with an antiandrogen in castrated patients with prostatic cancer. J. Clin. Endocrinol. Metab. 59, 422–426 (1984).
Nishii, M. et al. Luteinizing hormone (LH)-releasing hormone agonist reduces serum adrenal androgen levels in prostate cancer patients: implications for the effect of LH on the adrenal glands. J. Androl. 33, 1233–1238 (2012).
Bruchovsky, N. & Wilson, J. D. The conversion of testosterone to 5α-androstan-17β-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 243, 2012–2021 (1968).
Labrie, F., Luu-The, V., Labrie, C. & Simard, J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front. Neuroendocrinol. 22, 185–212 (2001).
Zaslavsky, A. B. et al. Platelet-synthesized testosterone in men with prostate cancer induces androgen receptor signaling. Neoplasia 17, 490–496 (2015).
Labrie, F. et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J. Steroid Biochem. Mol. Biol. 99, 182–188 (2006).
Attard, G. et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J. Clin. Endocrinol. Metab. 97, 507–516 (2012).
Locke, J. A. et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 68, 6407–6415 (2008).
Fankhauser, M. et al. Canonical androstenedione reduction is the predominant source of signaling androgens in hormone-refractory prostate cancer. Clin. Cancer Res. 20, 5547–5557 (2014).
Chang, K. H. et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 108, 13728–13733 (2011).
Penning, T. M. et al. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 351, 67–77 (2000).
Mostaghel, E. A. et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 17, 5913–5925 (2011).
van Weerden, W. M., Bierings, H. G., van Steenbrugge, G. J., de Jong, F. H. & Schroder, F. H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 50, 857–861 (1992).
Perkins, L. M. & Payne, A. H. Quantification of P450scc, 45017α, and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice. Endocrinology 123, 2675–2682 (1988).
Brock, B. J. & Waterman, M. R. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry 38, 1598–1606 (1999).
Luu-The, V., Pelletier, G. & Labrie, F. Quantitative appreciation of steroidogenic gene expression in mouse tissues: new roles for type 2 5α-reductase, 20α-hydroxysteroid dehydrogenase and estrogen sulfotransferase. J. Steroid Biochem. Mol. Biol. 93, 269–276 (2005).
Swerdloff, R. S., Wang, C. & Bhasin, S. Developments in the control of testicular function. Baillieres Clin. Endocrinol. Metab. 6, 451–483 (1992).
Dufau, M. L. et al. Regulation of androgen synthesis: the late steroidogenic pathway. Steroids 62, 128–132 (1997).
Payne, A. H., Youngblood, G. L., Sha, L., Burgos-Trinidad, M. & Hammond, S. H. Hormonal regulation of steroidogenic enzyme gene expression in Leydig cells. J. Steroid Biochem. Mol. Biol. 43, 895–906 (1992).
Hanukoglu, I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 43, 779–804 (1992).
Grist, E., de Bono, J. S. & Attard, G. Targeting extra-gonadal androgens in castration-resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 145, 157–163 (2015).
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Sharifi, N. Minireview: androgen metabolism in castration-resistant prostate cancer. Mol. Endocrinol. 27, 708–714 (2013).
Nakamura, Y. et al. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J. Clin. Endocrinol. Metab. 94, 2192–2198 (2009).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Armandari, I., Hamid, A. R., Verhaegh, G. & Schalken, J. Intratumoral steroidogenesis in castration-resistant prostate cancer: a target for therapy. Prostate Int. 2, 105–113 (2014).
Knudsen, K. E. Hormone whodunit: clues for solving the case of intratumor androgen production. Clin. Cancer Res. 20, 5343–5345 (2014).
Wilson, J. D. et al. 5α-androstane-3α, 17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α, 17α-diol-20-one as a key intermediate. Endocrinology 144, 575–580 (2003).
Auchus, R. J. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 15, 432–438 (2004).
Penning, T. M., Bauman, D. R., Jin, Y. & Rizner, T. L. Identification of the molecular switch that regulates access of 5α-DHT to the androgen receptor. Mol. Cell. Endocrinol. 265–266, 77–82 (2007).
Bauman, D. R., Steckelbroeck, S., Williams, M. V., Peehl, D. M. & Penning, T. M. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α, 17β-diol to 5α-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol. Endocrinol. 20, 444–458 (2006).
Mohler, J. L. et al. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res. 71, 1486–1496 (2011).
Shaw, G. et al. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α, 17β-diol. Proc. Natl Acad. Sci. USA 97, 12256–12259 (2000).
Locke, J. A. et al. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J. Steroid Biochem. Mol. Biol. 115, 126–136 (2009).
Kumagai, J. et al. Intratumoral conversion of adrenal androgen precursors drives androgen receptor-activated cell growth in prostate cancer more potently than de novo steroidogenesis. Prostate 73, 1636–1650 (2013).
Hofland, J. et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 70, 1256–1264 (2010).
Wu, Y. et al. Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5α-reductase inhibitors. Prostate 73, 1470–1482 (2013).
Sakai, M., Martinez-Arguelles, D. B., Aprikian, A. G., Magliocco, A. M. & Papadopoulos, V. De novo steroid biosynthesis in human prostate cell lines and biopsies. Prostate 76, 575–587 (2016).
Efstathiou, E. et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 67, 53–60 (2015).
Jernberg, E. et al. Characterization of prostate cancer bone metastases according to expression levels of steroidogenic enzymes and androgen receptor splice variants. PLoS ONE 8, e77407 (2013).
Campbell, T. J., Tindall, D. J. & Figg, W. D. Dihydrotestosterone synthesis from adrenal precursors does not involve testosterone in castration-resistant prostate cancer. Cancer Biol. Ther. 13, 237–238 (2012).
Titus, M. A. et al. Steroid 5α-reductase isozymes I and II in recurrent prostate cancer. Clin. Cancer Res. 11, 4365–4371 (2005).
Thomas, L. N. et al. Differential alterations in 5α-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate 63, 231–239 (2005).
Knudsen, K. E. & Penning, T. M. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 21, 315–324 (2010).
Jez, J. M., Flynn, T. G. & Penning, T. M. A new nomenclature for the aldo-keto reductase superfamily. Biochem. Pharmacol. 54, 639–647 (1997).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Ryan, C. J., Molina, A. & Griffin, T. Abiraterone in metastatic prostate cancer. N. Engl. J. Med. 368, 1458–1459 (2013).
Chen, E. J. et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin. Cancer Res. 21, 1273–1280 (2015).
Penning, T. M. Mechanisms of drug resistance that target the androgen axis in castration resistant prostate cancer (CRPC). J. Steroid Biochem. Mol. Biol. 153, 105–113 (2015).
Arakawa, H. et al. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochem. Pharmacol. 84, 1070–1077 (2012).
Thomas, M. P. & Potter, B. V. Estrogen O-sulfamates and their analogues: clinical steroid sulfatase inhibitors with broad potential. J. Steroid Biochem. Mol. Biol. 153, 160–169 (2015).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 349, 215–224 (2003).
Wilt, T. J., MacDonald, R., Hagerty, K., Schellhammer, P. & Kramer, B. S. Five-alpha-reductase Inhibitors for prostate cancer prevention. Cochrane Database Syst. Rev. 2, CD007091 (2008).
Walsh, P. C. Chemoprevention of prostate cancer. N. Engl. J. Med. 362, 1237–1238 (2010).
Andriole, G. L. et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 362, 1192–1202 (2010).
Opoku-Acheampong, A. B., Unis, D., Henningson, J. N., Beck, A. P. & Lindshield, B. L. Preventive and therapeutic efficacy of finasteride and dutasteride in TRAMP mice. PLoS ONE 8, e77738 (2013).
Shah, S. K. et al. Phase II study of dutasteride for recurrent prostate cancer during androgen deprivation therapy. J. Urol. 181, 621–626 (2009).
Chu, F. M. et al. A randomised, double-blind study comparing the addition of bicalutamide with or without dutasteride to GnRH analogue therapy in men with non-metastatic castrate-resistant prostate cancer. Eur. J. Cancer 51, 1555–1569 (2015).
Sartor, O. et al. Activity of dutasteride plus ketoconazole in castration-refractory prostate cancer after progression on ketoconazole alone. Clin. Genitourin. Cancer 7, E90–E92 (2009).
Taplin, M. E. et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin. Cancer Res. 15, 7099–7105 (2009).
Kikuchi, A. et al. In vitro and in vivo characterisation of ASP9521: a novel, selective, orally bioavailable inhibitor of 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5; AKR1C3). Invest. New Drugs 32, 860–870 (2014).
Loriot, Y. et al. Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest. New Drugs 32, 995–1004 (2014).
Kawabe, M. et al. Decrease of prostaglandin E2 and 5-bromo-2′-deoxyuridine labeling but not prostate tumor development by indomethacin treatment of rats given 3,2′-dimethyl-4-aminobiphenyl and testosterone propionate. Jpn. J. Cancer Res. 88, 350–355 (1997).
Hamid, A. R. et al. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol. Med. 18, 1449–1455 (2012).
Liedtke, A. J. et al. Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J. Med. Chem. 56, 2429–2446 (2013).
Evaul, K., Li, R., Papari-Zareei, M., Auchus, R. J. & Sharifi, N. 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology 151, 3514–3520 (2010).
Chang, K. H. et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013).
Wu, G. et al. Variant allele of HSD3B1 increases progression to castration-resistant prostate cancer. Prostate 75, 777–782 (2015).
Takizawa, I. et al. Trilostane, an inhibitor of 3β-hydroxysteroid dehydrogenase, has an agonistic activity on androgen receptor in human prostate cancer cells. Cancer Lett. 297, 226–230 (2010).
Geldof, A. A., Meulenbroek, M. F., Dijkstra, I., Bohlken, S. & Rao, B. R. Consideration of the use of 17 beta-N,N-diethylcarbamoyl-4-methyl-4-aza-5- alpha-androstan-3-one (4MA), a 5 alpha-reductase inhibitor, in prostate cancer therapy. J. Cancer Res. Clin. Oncol. 118, 50–55 (1992).
Li, R. et al. Abiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin. Cancer Res. 18, 3571–3579 (2012).
Li, Z. et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 523, 347–351 (2015).
Liden, M. & Eriksson, U. Understanding retinol metabolism: structure and function of retinol dehydrogenases. J. Biol. Chem. 281, 13001–13004 (2006).
Yamamoto, H. et al. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat. Genet. 22, 188–191 (1999).
Mitsiades, N. et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 72, 6142–6152 (2012).
Avila, D. M., Fuqua, S. A., George, F. W. & McPhaul, M. J. Identification of genes expressed in the rat prostate that are modulated differently by castration and Finasteride treatment. J. Endocrinol. 159, 403–411 (1998).
Hsiao, P. W., Thin, T. H., Lin, D. L. & Chang, C. Differential regulation of testosterone versus 5α-dihydrotestosterone by selective androgen response elements. Mol. Cell. Biochem. 206, 169–175 (2000).
Zhang, A., Zhang, J., Plymate, S. & Mostaghel, E. A. Classical and non-classical roles for pre-receptor control of DHT metabolism in prostate cancer progression. Horm. Cancer 7, 104–113 (2016).
Pfeiffer, M. J., Smit, F. P., Sedelaar, J. P. & Schalken, J. A. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol. Med. 17, 657–664 (2011).
Acknowledgements
The Australian Prostate Cancer Centre Epworth is supported by the Australian Government as represented by the Department of Health and Ageing. N.M.C. is supported by a David Bickart Clinician Research Fellowship from the Faculty of Medicine, Dentistry and Health Sciences at the University of Melbourne.
Author information
Authors and Affiliations
Contributions
R.S. and P.J.M. researched data for this article, all authors made a substantial contribution to discussions of content, R.S. and N.M.C. wrote the manuscript and N.M.C. and C.M.H. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Stuchbery, R., McCoy, P., Hovens, C. et al. Androgen synthesis in prostate cancer: do all roads lead to Rome?. Nat Rev Urol 14, 49–58 (2017). https://doi.org/10.1038/nrurol.2016.221
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.221
This article is cited by
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy
Nature Reviews Cancer (2023)
-
Orphan nuclear receptors as regulators of intratumoral androgen biosynthesis in castration-resistant prostate cancer
Oncogene (2021)
-
Unbiased data mining identifies cell cycle transcripts that predict non-indolent Gleason score 7 prostate cancer
BMC Urology (2019)
-
Glucocorticoids Induce Stress Oncoproteins Associated with Therapy-Resistance in African American and European American Prostate Cancer Cells
Scientific Reports (2018)