Key Points
-
The conventional serum protein tumour markers α-fetoprotein (AFP), human chorionic gonadotrophin (hCG) and lactate dehydrogenase (LDH) show utility in the management of testicular malignant germ cell tumours (GCTs)
-
AFP and hCG show limited sensitivity and specificity for all malignant GCTs, being representative of yolk sac tumour and choriocarcinoma or synctiotrophoblast subtypes, respectively; LDH is a very nonspecific biomarker
-
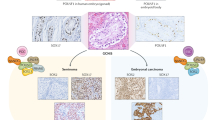
Novel universal biomarkers for testicular malignant GCTs are required, particularly for seminoma and embryonal carcinoma subtypes that are typically negative for conventional markers
-
MicroRNAs are short, non-protein-coding RNAs that show much promise as universal markers in malignant GCTs
-
Individual microRNAs from two microRNA clusters, miR-371–373 and miR-302–367, are overexpressed in all malignant GCTs, regardless of patient age, tumour site and subtype
-
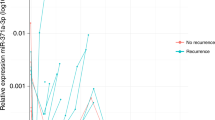
A panel of four circulating microRNAs from these two clusters (miR-371a-3p, miR-372-3p, miR-373-3p and miR-367-3p) is highly sensitive and specific for the diagnosis of malignant GCT, including seminoma and embryonal carcinoma subtypes
-
Practical considerations need to be addressed to standardize the translation of circulating microRNA studies from a research tool to a routine clinical test
Abstract
Testicular germ cell tumours (GCTs) are the most common malignancy occurring in young adult men and the incidence of these tumours is increasing. Current research priorities in this field include improving overall survival for patients classified as being 'poor-risk' and reducing late effects of treatment for patients classified as 'good-risk'. Testicular GCTs are broadly classified into seminomas and nonseminomatous GCTs (NSGCTs). The conventional serum protein tumour markers α-fetoprotein (AFP), human chorionic gonadotrophin (hCG) and lactate dehydrogenase (LDH) show some utility in the management of testicular malignant GCT. However, AFP and hCG display limited sensitivity and specificity, being indicative of yolk sac tumour (AFP) and choriocarcinoma or syncytiotrophoblast (hCG) subtypes. Furthermore, LDH is a very nonspecific biomarker. Consequently, seminomas and NSGCTs comprising a pure embryonal carcinoma subtype are generally negative for these conventional markers. As a result, novel universal biomarkers for testicular malignant GCTs are required. MicroRNAs are short, non-protein-coding RNAs that show much general promise as biomarkers. MicroRNAs from two 'clusters', miR-371–373 and miR-302–367, are overexpressed in all malignant GCTs, regardless of age (adult or paediatric), site (gonadal or extragonadal) and subtype (seminomas, yolk sac tumours or embryonal carcinomas). A panel of four circulating microRNAs from these two clusters (miR-371a-3p, miR-372-3p, miR-373-3p and miR-367-3p) is highly sensitive and specific for the diagnosis of malignant GCT, including seminoma and embryonal carcinoma. In the future, circulating microRNAs might be useful in diagnosis, disease monitoring and prognostication of malignant testicular GCTs, which might also reduce reliance on serial CT scanning. For translation into clinical practice, important practical considerations now need addressing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Trabert, B., Chen, J., Devesa, S. S., Bray, F. & McGlynn, K. A. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology 3, 4–12 (2015).
Moch, H., Humphrey, P. A., Ulbright, T. M. & Reuter, V. E. (eds) WHO/IARC Classification of Tumours of the Urinary System and Male Genital Organs 4th edn Vol. 8 (WHO, 2016).
Gilligan, T. D. et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 28, 3388–3404 (2010).
de Wit, R. & Fizazi, K. Controversies in the management of clinical stage I testis cancer. J. Clin. Oncol. 24, 5482–5492 (2006).
Cohn-Cedermark, G., Stahl, O., Tandstad, T. Surveillance versus adjuvant therapy of clinical stage I testicular tumors - a review and the SWENOTECA experience. Andrology 3, 102–110 (2015).
Daugaard, G. et al. Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. J. Clin. Oncol. 32, 3817–3823 (2014).
Gilbert, D. C. et al. Defining a new prognostic index for stage I non-seminomatous germ cell tumors using CXCL12 expression and proportion of embyronal carcinoma. Clin. Cancer Res. 22, 1265–1273 (2015).
Kobayashi, K. et al. Oncological outcomes in patients with stage I testicular seminoma and nonseminoma: pathological risk factors for relapse and feasibility of surveillance after orchiectomy. Diagn. Pathol. 8, 57 (2013).
Kollmannsberger, C. et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J. Clin. Oncol. 33, 51–57 (2015).
Lago-Hernandez, C. A. et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Ann. Oncol. 26, 1396–1401 (2015).
International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J. Clin. Oncol. 15, 594–603 (1997).
Murray, M. J. et al. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer 114, 151–162 (2016).
Dieckmann, K. P. et al. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br. J. Cancer 107, 1754–1760 (2012).
Tarin, T. V., Sonn, G. & Shinghal, R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J. Urol. 181, 627–632 (2009).
Fung, C., Fossa, S. D., Milano, M. T., Oldenburg, J. & Travis, L. B. Solid tumors after chemotherapy or surgery for testicular nonseminoma: a population-based study. J. Clin. Oncol. 31, 3807–3814 (2013).
Haugnes, H. S. et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J. Clin. Oncol. 30, 3752–3763 (2012).
Haugnes, H. S. et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J. Clin. Oncol. 28, 4649–4657 (2010).
Huddart, R. A. et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J. Clin. Oncol. 21, 1513–1523 (2003).
Kollmannsberger, C. et al. Late toxicity following curative treatment of testicular cancer. Semin. Surg. Oncol. 17, 275–281 (1999).
Murray, M. J., Nicholson, J. C. & Coleman, N. Biology of childhood germ cell tumours, focussing on the significance of microRNAs. Andrology 3, 129–139 (2015).
Travis, L. B. et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J. Natl Cancer Inst. 97, 1354–1365 (2005).
Murray, M. J. & Nicholson, J. C. α-Fetoprotein. Arch. Dis. Child. Educ. Pract. Ed. 96, 141–147 (2011).
Gitlin, D., Perricelli, A. & Gitlin, G. M. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 32, 979–982 (1972).
Nishizono, I. et al. Rapid and sensitive chemiluminescent enzyme immunoassay for measuring tumor markers. Clin. Chem. 37, 1639–1644 (1991).
Blohm, M. E., Vesterling-Horner, D., Calaminus, G. & Gobel, U. Alpha1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr. Hematol. Oncol. 15, 135–142 (1998).
Johnson, P. J. Role of alpha-fetoprotein in the diagnosis and management of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 14, S32–S36 (1999).
Schneider, D. T., Calaminus, G. & Gobel, U. Diagnostic value of alpha1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr. Hematol. Oncol. 18, 11–26 (2001).
Germa, J. R., Llanos, M., Tabernero, J. M. & Mora, J. False elevations of alpha-fetoprotein associated with liver dysfunction in germ cell tumors. Cancer 72, 2491–2494 (1993).
Murray, M. J. & Nicholson, J. C. Germ cell tumours in children and adolescents. Paediatr. Child Health 20, 109–116 (2010).
Murray, M. J. et al. Intra-abdominal metastasis of an intracranial germinoma via ventriculo-peritoneal shunt in a 13-year-old female. Br. J. Neurosurg. 25, 747–749 (2011).
Gilbert, D. C. et al. Clinical and biological significance of CXCL12 and CXCR4 expression in adult testes and germ cell tumours of adults and adolescents. J. Pathol. 217, 94–102 (2009).
Warde, P. et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J. Clin. Oncol. 20, 4448–4452 (2002).
Chung, P. et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 4, 155–160 (2015).
Miller, K. D., Loehrer, P. J., Gonin, R. & Einhorn, L. H. Salvage chemotherapy with vinblastine, ifosfamide, and cisplatin in recurrent seminoma. J. Clin. Oncol. 15, 1427–1431 (1997).
Vuky, J. et al. Salvage chemotherapy for patients with advanced pure seminoma. J. Clin. Oncol. 20, 297–301 (2002).
Raggi, D. et al. Prognostic reclassification of patients with intermediate-risk metastatic germ cell tumors: Implications for clinical practice, trial design, and molecular interrogation. Urol. Oncol. 33, 332.e19–332.e24 (2015).
van Dijk, M. R., Steyerberg, E. W., Stenning, S. P. & Habbema, J. D. Identifying subgroups among poor prognosis patients with nonseminomatous germ cell cancer by tree modelling: a validation study. Ann. Oncol. 15, 1400–1405 (2004).
Huddart, R. A. et al. A randomised phase 2 trial of intensive induction chemotherapy (CBOP/BEP) and standard BEP in poor-prognosis germ cell tumours (MRC TE23, CRUK 05/014, ISRCTN 53643604). Eur. Urol. 67, 534–543 (2015).
de Wit, R. et al. Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983. J. Clin. Oncol. 30, 792–799 (2012).
Daugaard, G. et al. A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974). Ann. Oncol. 22, 1054–1061 (2011).
Fizazi, K. et al. Early predicted time to normalization of tumor markers predicts outcome in poor-prognosis nonseminomatous germ cell tumors. J. Clin. Oncol. 22, 3868–3876 (2004).
Fizazi, K. et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol. 15, 1442–1450 (2014).
Killock, D. After 25 years, therapy for poor-prognosis GCTs advances. Nat. Rev. Clin. Oncol. 12, 3 (2015).
Lorch, A. A practice-changing step forward in germ-cell cancer? Lancet Oncol. 15, 1409–1410 (2014).
de Wit, R. et al. Serum alpha-fetoprotein surge after the initiation of chemotherapy for non-seminomatous testicular cancer has an adverse prognostic significance. Br. J. Cancer 78, 1350–1355 (1998).
Grigor, K. M., Detre, S. I., Kohn, J. & Neville, A. M. Serum alpha1-foetoprotein levels in 153 male patients with germ cell tumours. Br. J. Cancer 35, 52–58 (1977).
Ackers, C. & Rustin, G. J. Lactate dehydrogenase is not a useful marker for relapse in patients on surveillance for stage I germ cell tumours. Br. J. Cancer 94, 1231–1232 (2006).
Venkitaraman, R. et al. The utility of lactate dehydrogenase in the follow-up of testicular germ cell tumours. BJU Int. 100, 30–32 (2007).
Kawakami, T., Okamoto, K., Ogawa, O. & Okada, Y. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet 363, 40–42 (2004).
Rainen, L. et al. Stabilization of mRNA expression in whole blood samples. Clin. Chem. 48, 1883–1890 (2002).
Viprey, V. F. et al. Standardisation of operating procedures for the detection of minimal disease by QRT-PCR in children with neuroblastoma: quality assurance on behalf of SIOPEN-R-NET. Eur. J. Cancer 43, 341–350 (2007).
Bachner, M. et al. 2-18Fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann. Oncol. 23, 59–64 (2012).
De Santis, M. et al. 2-18Fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J. Clin. Oncol. 22, 1034–1039 (2004).
Hain, S. F. et al. Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. Br. J. Cancer 83, 863–869 (2000).
Huddart, R. A. et al. 18Fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: preliminary report of MRC Trial TE22—the NCRI Testis Tumour Clinical Study Group. J. Clin. Oncol. 25, 3090–3095 (2007).
Voorhoeve, P. M. et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124, 1169–1181 (2006).
Gillis, A. J. M. et al. High-throughput microRNAome analysis in human germ cell tumours. J. Pathol. 213, 319–328 (2007).
Palmer, R. D. et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 70, 2911–2923 (2010).
Bing, Z. et al. MicroRNA expression profiles of seminoma from paraffin-embedded formalin-fixed tissue. Virchows Arch. 461, 663–668 (2012).
Novotny, G. W. et al. MicroRNA expression profiling of carcinoma in situ cells of the testis. Endocr. Relat. Cancer 19, 365–379 (2012).
Caby, M. P., Lankar, D., Vincendeau-Scherrer, C., Raposo, G. & Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887 (2005).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Murray, M. J. et al. Identification of MicroRNAs from the miR-371∼373 and miR-302 clusters as potential serum biomarkers of malignant germ cell tumors. Am. J. Clin. Pathol. 135, 119–125 (2011).
Murray, M. J. & Coleman, N. Testicular cancer: a new generation of biomarkers for malignant germ cell tumours. Nat. Rev. Urol. 9, 298–300 (2012).
Belge, G., Dieckmann, K. P., Spiekermann, M., Balks, T. & Bullerdiek, J. Serum levels of microRNAs miR-371-3: a novel class of serum biomarkers for testicular germ cell tumors? Eur. Urol. 61, 1068–1069 (2012).
Gillis, A. J. M. et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: a proof of principle. Mol. Oncol. 7, 1083–1092 (2013).
Rijlaarsdam, M. A. et al. Identification of known and novel germ cell cancer-specific (embryonic) miRs in serum by high-throughput profiling. Andrology 3, 85–91 (2015).
Spiekermann, M. et al. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology 3, 78–84 (2015).
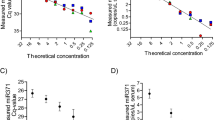
Spiekermann, M., Dieckmann, K. P., Balks, T., Bullerdiek, J. & Belge, G. Is relative quantification dispensable for the measurement of microRNAs as serum biomarkers in germ cell tumors? Anticancer Res. 35, 117–121 (2015).
Syring, I. et al. Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer. J. Urol. 193, 331–337 (2015).
Biofluids guidelines: analyzing microRNAs in liquid biopsies. Exiqon http://www.exiqon.com/ls/Documents/Scientific/microRNA-serum-plasma-guidelines.pdf (2015).
Litchfield, K. et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 6, 5973 (2015).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
van Agthoven T. & Looijenga L. H. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget http://dx.doi.org/10.18632/oncotarget.10867 (2016).
Dieckmann K. P et al. Serum levels of microRNA miR-371a-3p: a sensitive and specific new biomarker for germ cell tumours. Eurol. Urol. http://dx.doi.org/10.1016/j.eururo.2016.07.029 (2016)
Acknowledgements
The authors would like to acknowledge grant funding from CwCUK/GOSHCC (grant W1058 to M.J.M. and N.C.), SPARKS (grant 11CAM01 to M.J.M. and N.C.), CRUK (grant A13080 to N.C.) MRC (grant MC_EX_G0800464 to M.J.M.) and National Health Service funding to the Royal Marsden/Institute of Cancer Research National Institute for Health Research Biomedical Research Centre for Cancer (R.A.H.). The authors also thank the Max Williamson Fund, the Josh Carrick Foundation and The Perse Preparatory School, Cambridge for support.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made a substantial contribution to discussions of content, wrote and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
M.J.M. and N.C. receive laboratory funding from AstraZeneca as part of a collaborative research programme investigating clinical applications of circulating microRNA quantification. R.A.H. declares no competing interests.
Related links
Rights and permissions
About this article
Cite this article
Murray, M., Huddart, R. & Coleman, N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol 13, 715–725 (2016). https://doi.org/10.1038/nrurol.2016.170
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2016.170
This article is cited by
-
Serum tumour markers for testicular cancer recurrence
Nature Reviews Urology (2023)
-
Treatment de-escalation for stage II seminoma
Nature Reviews Urology (2023)
-
Two decades of FDG-PET/CT in seminoma: exploring its role in diagnosis, surveillance and follow-up
Cancer Imaging (2022)
-
Advancing clinical and translational research in germ cell tumours (GCT): recommendations from the Malignant Germ Cell International Consortium
British Journal of Cancer (2022)
-
Associations of serum levels of microRNA-371a-3p (M371) with risk factors for progression in nonseminomatous testicular germ cell tumours clinical stage 1
World Journal of Urology (2022)