Key Points
-
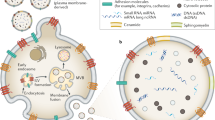
Extracellular vesicles are small (40–5,000 nm diameter) membrane-bound vesicles that can be categorized into exosomes, microvesicles and apoptotic bodies according to their size, origin, morphology and mode of release
-
Whereas the generation of exosomes involves endocytosis, formation of multivesicular bodies and subsequent membrane fusion, microvesicles are produced by membrane budding and apoptotic bodies result from membrane blebbing during apoptosis
-
Over the past 10 years, various methodologies for the effective isolation of extracellular vesicles have been developed, including centrifugation, affinity capture, precipitation and the use of microfluidic devices
-
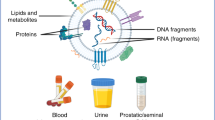
Extracellular vesicle cargo is thought to reflect the cell-type of origin, suggesting it could be a promising source for the discovery of novel biomarkers
Abstract
The knowledge gained from comprehensive profiling projects that aim to define the complex genomic alterations present within cancers will undoubtedly improve our ability to detect and treat those diseases, but the influence of these resources on our understanding of basic cancer biology is still to be demonstrated. Extracellular vesicles have gained considerable attention in past years, both as mediators of intercellular signalling and as potential sources for the discovery of novel cancer biomarkers. In general, research on extracellular vesicles investigates either the basic mechanism of vesicle formation and cargo incorporation, or the isolation of vesicles from available body fluids for biomarker discovery. A deeper understanding of the cargo molecules present in extracellular vesicles obtained from patients with urogenital cancers, through high-throughput proteomics or genomics approaches, will aid in the identification of novel diagnostic and prognostic biomarkers, and can potentially lead to the discovery of new therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Verma, S., Bhavsar, A. S. & Donovan, J. MR imaging-guided prostate biopsy techniques. Magn. Reson. Imaging Clin. N. Am. 22, 135–144 (2014).
Griffiths, T. R. & Action on Bladder Cancer. Current perspectives in bladder cancer management. Int. J. Clin. Pract. 67, 435–448 (2013).
Ljungberg, B. et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur. Urol. 58, 398–406 (2010).
Akers, J. C., Gonda, D., Kim, R., Carter, B. S. & Chen, C. C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 113, 1–11 (2013).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. http://dx.doi.org/10.1002/mas.21420.
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Théry, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.20360.
Nawaz, M. et al. Microvesicles in gliomas and medulloblastomas: an overview. J. Cancer Ther. 5, 182–191 (2014).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Camussi, G., Deregibus, M. C. & Tetta, C. Tumor-derived microvesicles and the cancer microenvironment. Curr. Mol. Med. 13, 58–67 (2013).
Grange, C. et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356 (2011).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Taylor, D. D., Lyons, K. S. & Gercel-Taylor, C. Shed membrane fragment-associated markers for endometrial and ovarian cancers. Gynecol. Oncol. 84, 443–448 (2002).
van Doormaal, F. F., Kleinjan, A., Di Nisio, M., Büller, H. R. & Nieuwland, R. Cell-derived microvesicles and cancer. Neth. J. Med. 67, 266–273 (2009).
Buzas, E. I., György, B., Nagy, G., Falus, A. & Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 (2014).
D'Souza-Schorey, C. & Clancy, J. W. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 26, 1287–1299 (2012).
György, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
Shedden, K., Xie, X. T., Chandaroy, P., Chang, Y. T. & Rosania, G. R. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 63, 4331–4337 (2003).
Théry, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Aalberts, M., Stout, T. A. & Stoorvogel, W. Prostasomes: extracellular vesicles from the prostate. Reproduction 147, R1–R14 (2014).
Chen, C. L. et al. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J. Proteome Res. 11, 5611–5629 (2012).
Drake, R. R. & Kislinger, T. The proteomics of prostate cancer exosomes. Expert Rev. Proteomics 11, 167–177 (2014).
Peng, P., Yan, Y. & Keng, S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol. Rep. 25, 749–762 (2011).
Poliakov, A., Spilman, M., Dokland, T., Amling, C. L. & Mobley, J. A. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 69, 159–167 (2009).
Principe, S. et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics 13, 1667–1671 (2013).
Smalley, D. M., Sheman, N. E., Nelson, K. & Theodorescu, D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J. Proteome Res. 7, 2088–2096 (2008).
Kosaka, N., Iguchi, H. & Ochiya, T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 101, 2087–2092 (2010).
Zernecke, A. et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2, ra81 (2009).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl Acad. Sci. USA 105, 10513–10518 (2008).
Sharkey, J. W., Antoine, D. J. & Park, B. K. Validation of the isolation and quantification of kidney enriched miRNAs for use as biomarkers. Biomarkers 17, 231–239 (2012).
Yu, Z. & Hecht, N. B. The DNA/RNA-binding protein, translin, binds microRNA122a and increases its in vivo stability. J. Androl. 29, 572–579 (2008).
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D. & Remaley, A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 13, 423–433 (2011).
Wang, K., Zhang, S., Weber, J., Baxter, D. & Galas, D. J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 38, 7248–7259 (2010).
Khalyfa, A. & Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 12, 162 (2014).
Graves, L. E. et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 64, 7045–7049 (2004).
Kim, H. K. et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur. J. Cancer 39, 184–191 (2003).
Kobayashi, M. et al. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J. Transl. Med. 12, 4 (2014).
Taylor, D. D. & Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21 (2008).
Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 (2009).
de Gassart, A., Géminard, C., Hoekstra, D. & Vidal, M. Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic 5, 896–903 (2004).
Hurley, J. H. & Odorizzi, G. Get on the exosome bus with ALIX. Nat. Cell Biol. 14, 654–655 (2012).
Baietti, M. F. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685 (2012).
Babst, M., Katzmann, D. J., Snyder, W. B., Wendland, B. & Emr, S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3, 283–289 (2002).
Katzmann, D. J., Stefan, C. J., Babst, M. & Emr, S. D. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 162, 413–423 (2003).
van Niel, G., Porto-Carreiro, I., Simoes, S. & Raposo, G. Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21 (2006).
Wollert, T. & Hurley, J. H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864–869 (2010).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Nickel, W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic 6, 607–614 (2005).
Hsu, C. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 (2010).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010).
Vanlandingham, P. A. & Ceresa, B. P. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 284, 12110–12124 (2009).
Del Conde, I., Shrimpton, C. N., Thiagarajan, P. & López, J. A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604–1611 (2005).
Nabhan, J. F., Hu, R., Oh, R. S., Cohen, S. N. & Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl Acad. Sci. USA 109, 4146–4151 (2012).
Bianco, F. et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054 (2009).
Muralidharan-Chari, V. et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885 (2009).
D'Souza-Schorey, C. & Chavrier, P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358 (2006).
Donaldson, J. G. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 278, 41573–41576 (2003).
Muralidharan-Chari, V. et al. ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res. 69, 2201–2209 (2009).
Li, B., Antonyak, M. A., Zhang, J. & Cerione, R. A. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31, 4740–4749 (2012).
Charras, G. T., Hu, C. K., Coughlin, M. & Mitchison, T. J. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175, 477–490 (2006).
Gadea, G., de Toledo, M., Anguille, C. & Roux, P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J. Cell Biol. 178, 23–30 (2007).
Rankin, K. E. & Wordeman, L. Long astral microtubules uncouple mitotic spindles from the cytokinetic furrow. J. Cell Biol. 190, 35–43 (2010).
Saraste, A. & Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 45, 528–537 (2000).
Bergsmedh, A. et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl Acad. Sci. USA 98, 6407–6411 (2001).
Alvarez, M. L., Khosroheidari, M., Kanchi Ravi, R. & DiStefano, J. K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 82, 1024–1032 (2012).
Kalra, H. et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 13, 3354–3364 (2013).
Rekker, K. et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 47, 135–138 (2014).
Tauro, B. J. et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56, 293–304 (2012).
Taylor, D. D., Zacharias, W. & Gercel-Taylor, C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol. 728, 235–246 (2011).
Yamada, T., Inoshima, Y., Matsuda, T. & Ishiguro, N. Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci. 74, 1523–1525 (2012).
Cvjetkovic, A., Lötvall, J. & Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 3 (2014).
Momen-Heravi, F. et al. Alternative methods for characterization of extracellular vesicles. Front. Physiol. 3, 354 (2012).
Momen-Heravi, F. et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 3, 162 (2012).
Cheruvanky, A. et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Renal Physiol. 292, F1657–F1661 (2007).
Merchant, M. L. et al. Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin. Appl. 4, 84–96 (2010).
Chen, T. S. et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 9, 47 (2011).
Lai, R. C. et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics 2012, 971907 (2012).
Taylor, D. D., Chou, I. N. & Black, P. H. Isolation of plasma membrane fragments from cultured murine melanoma cells. Biochem. Biophys. Res. Commun. 113, 470–476 (1983).
Clayton, A. et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods 247, 163–174 (2001).
Chen, C. et al. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10, 505–511 (2010).
Garcia-Contreras, M. & Robbins, P. D. Exosomes and microvesicles: applications for translational research from biomarkers to therapeutic applications—2013 ASMEV meeting report. CellR4 1, e412 (2013).
Hill, A. F. et al. ISEV position paper: extracellular vesicle RNA analysis and bioinformatics. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.22859 (2013).
Llorente, A. et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta 1831, 1302–1309 (2013).
Aras, O. et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood 103, 4545–4553 (2004).
Yuana, Y. et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.21494 (2013).
Sharma, S., Gillespie, B. M., Palanisamy, V. & Gimzewski, J. K. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir 27, 14394–14400 (2011).
Sharma, S. et al. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 4, 1921–1926 (2010).
Dragovic, R. A. et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 7, 780–788 (2011).
Gardiner, C., Ferreira, Y. J., Dragovic, R. A., Redman, C. W. & Sargent, I. L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2 (2013).
Sokolova, V. et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 87, 146–150 (2011).
Nolte-'t Hoen, E. N. et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine 8, 712–720 (2012).
Jørgensen, M. et al. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles http://dx.doi.org/10.3402/jev.v2i0.20920 (2013).
Logozzi, M. et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 4, e5219 (2009).
Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 32, 490–495 (2014).
Shao, H. et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 18, 1835–1840 (2012).
Yoshioka, Y. et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 5, 3591 (2014).
Slatkoff, S., Gamboa, S., Zolotor, A. J., Mounsey, A. L. & Jones, K. PURLs: PSA testing: when it's useful, when it's not. J. Fam. Pract. 60, 357–360 (2011).
Ronquist, G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J. Intern. Med. 271, 400–413 (2012).
Abouelleil, M., Bratslavsky, G. & Bourboulia, D. Prostate cancer biomarkers–a bench to bedside perspective. Cancer Sci. Res. Open Access 1, 3 (2014).
Vlaeminck-Guillem, V., Ruffion, A., André, J., Devonec, M. & Paparel, P. Urinary prostate cancer 3 test: toward the age of reason? Urology 75, 447–453 (2010).
Dhir, R. et al. Early identification of individuals with prostate cancer in negative biopsies. J. Urol. 171, 1419–1423 (2004).
Paul, B., Dhir, R., Landsittel, D., Hitchens, M. R. & Getzenberg, R. H. Detection of prostate cancer with a blood-based assay for early prostate cancer antigen. Cancer Res. 65, 4097–4100 (2005).
Perry, A. S., Furusato, B., Nagle, R. B. & Ghosh, S. Increased aPKC expression correlates with prostatic adenocarcinoma gleason score and tumor stage in the Japanese population. Prostate Cancer 2014, 481697 (2014).
Di Vizio, D. et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181, 1573–1584 (2012).
Gonzalgo, M. L., Pavlovich, C. P., Lee, S. M. & Nelson, W. G. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin. Cancer Res. 9, 2673–2677 (2003).
Hosseini-Beheshti, E., Pham, S., Adomat, H., Li, N. & Tomlinson Guns, E. S. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol. Cell. Proteomics 11, 863–885 (2012).
Mitchell, P. J. et al. Can urinary exosomes act as treatment response markers in prostate cancer? J. Transl. Med. 7, 4 (2009).
Nilsson, J. et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br. J. Cancer 100, 1603–1607 (2009).
Stamey, T. A. et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 317, 909–916 (1987).
Tavoosidana, G. et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc. Natl Acad. Sci. USA 108, 8809–8814 (2011).
Uetsuki, H. et al. Expression of a novel biomarker, EPCA, in adenocarcinomas and precancerous lesions in the prostate. J. Urol. 174, 514–518 (2005).
Utleg, A. G. et al. Proteomic analysis of human prostasomes. Prostate 56, 150–161 (2003).
Zielie, P. J. et al. A novel diagnostic test for prostate cancer emerges from the determination of alpha-methylacyl-coenzyme a racemase in prostatic secretions. J. Urol. 172, 1130–1133 (2004).
Gabriel, K. et al. Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PLoS ONE 8, e70047 (2013).
Khan, S. et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS ONE 7, e46737 (2012).
Huang, X., Liang, M., Dittmar, R. & Wang, L. Extracellular microRNAs in urologic malignancies: chances and challenges. Int. J. Mol. Sci. 14, 14785–14799 (2013).
Gallo, A., Tandon, M., Alevizos, I. & Illei, G. G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 7, e30679 (2012).
Bryant, R. J. et al. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 106, 768–774 (2012).
Brase, J. C. et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 128, 608–616 (2011).
Ronquist, K. G., Ronquist, G., Larsson, A. & Carlsson, L. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Res. 30, 285–290 (2010).
Duijvesz, D. et al. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS ONE 8, e82589 (2013).
Jansen, F. H. et al. Exosomal secretion of cytoplasmic prostate cancer xenograft-derived proteins. Mol. Cell. Proteomics 8, 1192–1205 (2009).
Sandvig, K. & Llorente, A. Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol. Cell. Proteomics 11, M111.012914 (2012).
Ronquist, G. & Nilsson, B. O. The Janus-faced nature of prostasomes: their pluripotency favours the normal reproductive process and malignant prostate growth. Prostate Cancer Prostatic Dis. 7, 21–31 (2004).
Nyalwidhe, J. O. et al. Increased bisecting N-acetylglucosamine and decreased branched chain glycans of N-linked glycoproteins in expressed prostatic secretions associated with prostate cancer progression. Proteomics Clin. Appl. 7, 677–689 (2013).
Bijnsdorp, I. V. et al. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles 2 (2013).
Lu, Q. et al. Identification of extracellular delta-catenin accumulation for prostate cancer detection. Prostate 69, 411–418 (2009).
Pisitkun, T., Shen, R. F. & Knepper, M. A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl Acad. Sci. USA 101, 13368–13373 (2004).
Zhou, H. et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 69, 1471–1476 (2006).
Dijkstra, S. et al. Prostate cancer biomarker profiles in urinary sediments and exosomes. J. Urol. 191, 1132–1138 (2014).
Rathmell, W. K. & Godley, P. A. Recent updates in renal cell carcinoma. Curr. Opin. Oncol. 22, 250–256 (2010).
Lopez-Beltran, A. et al. 2009 update on the classification of renal epithelial tumors in adults. Int. J. Urol. 16, 432–443 (2009).
Bausch, B. et al. Renal cancer in von Hippel-Lindau disease and related syndromes. Nat. Rev. Nephrol. 9, 529–538 (2013).
Grange, C., Collino, F., Tapparo, M. & Camussi, G. Oncogenic micro-RNAs and renal cell carcinoma. Front. Oncol. 4, 49 (2014).
Wulfken, L. M. et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS ONE 6, e25787 (2011).
Redova, M. et al. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J. Transl. Med. 10, 55 (2012).
Hauser, S. et al. Analysis of serum microRNAs (miR-26a-2*, miR-191, miR-337-3p and miR-378) as potential biomarkers in renal cell carcinoma. Cancer Epidemiol. 36, 391–394 (2012).
Zhao, A., Li, G., Péoc'h, M., Genin, C. & Gigante, M. Serum miR-210 as a novel biomarker for molecular diagnosis of clear cell renal cell carcinoma. Exp. Mol. Pathol. 94, 115–120 (2013).
von Brandenstein, M. et al. MicroRNA 15a, inversely correlated to PKCα, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am. J. Pathol. 180, 1787–1797 (2012).
Chow, T. F. et al. Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin. Biochem. 43, 150–158 (2010).
Yang, L., Wu, X., Wang, D., Luo, C. & Chen, L. Renal carcinoma cell-derived exosomes induce human immortalized line of Jurkat T lymphocyte apoptosis in vitro. Urol. Int. 91, 363–369 (2013).
Del Boccio, P. et al. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: application to RCC urinary exosomes. Electrophoresis 33, 689–696 (2012).
Raimondo, F. et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Biosyst. 9, 1220–1233 (2013).
Schrier, B. P., Hollander, M. P., van Rhijn, B. W., Kiemeney, L. A. & Witjes, J. A. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur. Urol. 45, 292–296 (2004).
Fradet, Y. & Lockhard, C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt™. Can. J. Urol. 4, 400–405 (1997).
Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. 26, 646–651 (2008).
Landman, J., Chang, Y., Kavaler, E., Droller, M. J. & Liu, B. C. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology 52, 398–402 (1998).
Xylinas, E. et al. Urine markers for detection and surveillance of bladder cancer. Urol. Oncol. 32, 222–229 (2014).
Sharma, S., Zippe, C. D., Pandrangi, L., Nelson, D. & Agarwal, A. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J. Urol. 162, 53–57 (1999).
Lin, H. Y. et al. p53 codon 72 polymorphism as a progression index for bladder cancer. Oncol. Rep. 27, 1193–1199 (2012).
Kamat, A. M. & Mathew, P. Bladder cancer: imperatives for personalized medicine. Oncology (Williston Park) 25, 951–958, 960 (2011).
Park, H. S. et al. Quantitation of Aurora kinase A gene copy number in urine sediments and bladder cancer detection. J. Natl Cancer Inst. 100, 1401–1411 (2008).
Welton, J. L. et al. Proteomics analysis of bladder cancer exosomes. Mol. Cell. Proteomics 9, 1324–1338 (2010).
Beckham, C. J. et al. Bladder cancer exosomes contain EDIL-3/Del1 and Facilitate cancer progression. J. Urol. 192, 583–592 (2014).
Perez, A. et al. A pilot study on the potential of RNA-associated to urinary vesicles as a suitable non-invasive source for diagnostic purposes in bladder cancer. Cancers (Basel) 6, 179–192 (2014).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13, 1554–1571 (2013).
Cossetti, C. et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell http://dx.doi.org/10.1016/j.molcel.2014.08.020.
Liu, T., Mendes, D. E. & Berkman, C. E. Functional prostate-specific membrane antigen is enriched in exosomes from prostate cancer cells. Int. J. Oncol. 44, 918–922 (2014).
Ludwig, J. A. & Weinstein, J. N. Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 5, 845–856 (2005).
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L. & Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420 (1987).
Théry, C., Amigorena, S., Raposo G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. http://dx.doi.org/10.1002/0471143030.cb0322s30 (2006).
Burns, G. et al. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 9, e90913 (2014).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Nolte-'t Hoen, E. N. et al. Dynamics of dendritic cell-derived vesicles: high-resolution flow cytometric analysis of extracellular vesicle quantity and quality. J. Leukoc. Biol. 93, 395–402 (2013).
Acknowledgements
This work was supported by a Movember Discovery grant from Prostate Cancer Canada (D2013-21) and by the Canada Research Chair Program to T. Kislinger. M. Nawaz, F. Fatima and L. Neder acknowledge funding from FAPESP (Sao Paulo Research Foundation, Proc. No. 12/24574-3) and CAPES (Coordination for the Improvement of Higher Education Personnel). K. Ekström and X. Wang are supported by The Swedish Research Council (K2012-52X-09495-25-3), the BIOMATCELL VINN Excellence Center of Biomaterials and Cell Therapy, the Stiftelsen Handlanden Hjalmar Svensson Foundation and the Magnus Bergvalls Foundation. G. Camussi acknowledges funding from Associazione Italiana per la Ricerca sul Cancro (AIRC) IG2012 n. 12890.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to discussions of content, writing the article and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nawaz, M., Camussi, G., Valadi, H. et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol 11, 688–701 (2014). https://doi.org/10.1038/nrurol.2014.301
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2014.301
This article is cited by
-
Localized DNA tetrahedrons assisted catalytic hairpin assembly for the rapid and sensitive profiling of small extracellular vesicle-associated microRNAs
Journal of Nanobiotechnology (2022)
-
Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer
BMC Cancer (2022)
-
Circulating exosomal mRNA signatures for the early diagnosis of clear cell renal cell carcinoma
BMC Medicine (2022)
-
Urinary extracellular vesicle mRNA analysis of sodium chloride cotransporter in hypertensive patients under different conditions
Journal of Human Hypertension (2022)
-
Tumor-derived exosomal non-coding RNAs as diagnostic biomarkers in cancer
Cellular and Molecular Life Sciences (2022)