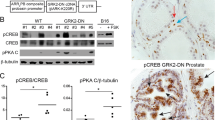
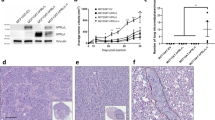
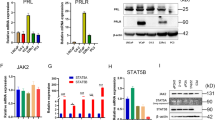
Abstract
Prolactin is best known for its actions on the mammary gland. However, circulating prolactin is also detected in males and its receptor (PRLR) is expressed in the prostate, suggesting that the prostate is a target of prolactin. Germline knockout of prolactin or its receptor has failed to reveal a key role for prolactin signaling in mouse prostate physiology. However, several studies involving rodent models and human prostate cell lines and specimens have supported the contribution of the canonical PRLR–Jak2–Stat5a/b pathway to prostate cancer tumorigenesis and progression. Increased expression of prolactin in the prostate itself (rather than changes in circulating prolactin levels) and crosstalk with androgen receptor (AR) signaling are potential mechanisms for increased Stat5a/b signaling in prostate cancer. In the mouse prostate, prolactin overexpression results in disorganized expansion of the basal/stem cell compartment, which has been proposed to house putative prostate tumor-initiating cells. These findings provide new insight into the molecular and cellular targets by which locally produced prolactin could contribute to prostate cancer initiation and progression. A number of pharmacological inhibitors targeting various levels of the PRLR–Jak2–Stat5a/b pathway have been developed and are entering clinical trials for advanced prostate cancer.
Key Points
-
Phenotypic analysis of prolactin or prolactin receptor (PRLR) deficient mice suggest that PRLR signaling moderately contributes to prostate development and physiology, but corresponding data for humans are currently lacking
-
Involvement of prolactin in benign prostate diseases has been proposed, based on the identification of such phenotypes in prolactin-overexpressing rodent models; however, the corresponding evidence is outstanding for humans
-
Circulating prolactin levels do not correlate with prostate cancer risk in humans; however, overactivation of PRLR signaling by increased prostatic expression of prolactin might contribute to prostate cancer progression
-
Putative tumor-initiating cells localize within the basal/stem cell compartment in the prostate epithelium—the histological phenotype of prostate tumors developed by prolactin-overexpressing mice involves disorganized amplification of this compartment
-
Stat5a/b is a primary mediator of prolactin effects in prostate epithelium; Stat5a/b is critical in prostate cancer progression, and inhibition of this pathway leads to widespread apoptosis
-
The PRLR, its associated kinase Jak2, or the transcription factor Stat5a/b offer potential targets for pharmaceutical intervention; proof-of-concept in experimental models has been obtained for various inhibitors
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Riddle, O., Bates, R. W. & Dykshorn, S. W. The preparation, identification and assay of prolactin—a hormone of the anterior pituitary. Am. J. Physiol. 105, 191–216 (1933).
Kelly, P. A., Djiane, J., Postel-Vinay, M. C. & Edery, M. The prolactin/growth hormone receptor family. Endocr. Rev. 12, 235–251 (1991).
Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P. A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 (1998).
Ormandy, C. J. et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 11, 167–178 (1997).
Horseman, N. D. et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16, 6926–6935 (1997).
Liu, X. et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186 (1997).
Ben-Jonathan, N., LaPensee, C. R. & LaPensee, E. W. What can we learn from rodents about prolactin in humans? Endocr. Rev. 29, 1–41 (2008).
Ramot, Y. et al. Prolactin—a novel neuroendocrine regulator of human keratin expression in situ. FASEB J. 24, 1768–1779 (2010).
LaPensee, C. R. et al. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147, 4638–4645 (2006).
Dorshkind, K. & Horseman, N. D. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr. Rev. 21, 292–312 (2000).
Flores, A., Segaloff, A. & Steelman, S. L. Prolactin as a factor in the ventral prostate assay for luteinizing hormone. Endocrinology 59, 233–240 (1956).
Costello, L. C. & Franklin, R. B. Effect of prolactin on the prostate. Prostate 24, 162–166 (1994).
Nevalainen, M. T. et al. Prolactin and prolactin receptors are expressed and functioning in human prostate. J. Clin. Invest. 99, 618–627 (1997).
Newball, H. H. & Byar, D. P. Does reserpine increase prolactin and exacerbate cancer of prostate? Case control study. Urology 2, 525–529 (1973).
Rouet, V. et al. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc. Natl Acad. Sci. USA 107, 15199–15204 (2010).
Goldstein, A. S., Huang, J., Guo, C., Garraway, I. P. & Witte, O. N. Identification of a cell of origin for human prostate cancer. Science 329, 568–571 (2010).
Walvoord, D. J., Resnick, M. I. & Grayhack, J. T. Effect of testosterone, dihydrotestosterone, estradiol, and prolactin on the weight and citric acid content of the lateral lobe of the rat prostate. Invest. Urol. 14, 60–65 (1976).
Coert, A., Nievelstein, H., Kloosterboer, H. J., Loonen, P. & van deer Vies, J. Effects of hyperprolactinemia on the accessory sexual organs of the male rat. Prostate 6, 269–276 (1985).
Jones, R., Riding, P. R. & Parker, M. G. Effects of prolactin on testosterone-induced growth and protein synthesis in rat accessory sex glands. J. Endocrinol. 96, 407–416 (1983).
Holland, J. M. & Lee, C. Effects of pituitary grafts on testosterone stimulated growth of rat prostate. Biol. Reprod. 22, 351–355 (1980).
Thomas, J. A. & Manandhar, M. S. Effects of prolactin on the dorsolateral lobe of the rat prostate gland. Invest. Urol. 14, 398–399 (1977).
Schacht, M. J. et al. A local direct effect of pituitary graft on growth of the lateral prostate in rats. Prostate 20, 51–58 (1992).
Keenan, E. J., Klase, P. A. & Thomas, J. A. Effects of prolactin on DNA synthesis and growth of the accessory sex organs in male mice. Endocrinology 109, 170–175 (1981).
Prins, G. S. Prolactin influence on cytosol and nuclear androgen receptors in the ventral, dorsal, and lateral lobes of the rat prostate. Endocrinology 120, 1457–1464 (1987).
Adams, J. B. Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland. Mol. Cell Endocrinol. 41, 1–17 (1985).
Nevalainen, M. T. et al. Estrogen and prolactin regulation of rat dorsal and lateral prostate in organ culture. Endocrinology 129, 612–622 (1991).
Ahonen, T. J. et al. Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology 140, 5412–5421 (1999).
Ahonen, T. J., Harkonen, P. L., Rui, H. & Nevalainen, M. T. PRL signal transduction in the epithelial compartment of rat prostate maintained as long-term organ cultures in vitro. Endocrinology 143, 228–238 (2002).
Li, H. et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 64, 4774–4782 (2004).
Dagvadorj, A. et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology 148, 3089–3101 (2007).
Reiter, E. et al. Growth hormone and prolactin stimulate androgen receptor, insulin-like growth factor-I (IGF-I) and IGF-I receptor levels in the prostate of immature rats. Mol. Cell Endocrinol. 88, 77–87 (1992).
Steger, R. W., Chandrashekar, V., Zhao, W., Bartke, A. & Horseman, N. D. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology 139, 3691–3695 (1998).
Robertson, F. G. et al. Prostate development and carcinogenesis in prolactin receptor knockout mice. Endocrinology 144, 3196–3205 (2003).
Nevalainen, M. T. et al. Epithelial defect in prostates of Stat5a-null mice. Lab. Invest. 80, 993–1006 (2000).
Abate-Shen, C. & Shen, M. M. Molecular genetics of prostate cancer. Genes Dev. 14, 2410–2434 (2000).
McNeal, J. E. The zonal anatomy of the prostate. Prostate 2, 35–49 (1981).
Wennbo, H., Kindblom, J., Isaksson, O. G. & Tornell, J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology 138, 4410–4415 (1997).
Kindblom, J. et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology 144, 2269–2278 (2003).
Kindblom, J., Dillner, K., Ling, C., Tornell, J. & Wennbo, H. Progressive prostate hyperplasia in adult prolactin transgenic mice is not dependent on elevated serum androgen levels. Prostate 53, 24–33 (2002).
Van Coppenolle, F. et al. Effects of hyperprolactinemia on rat prostate growth: evidence of androgeno-dependence. Am. J. Physiol. Endocrinol. Metab. 280, E120–E129 (2001).
Syms, A. J., Harper, M. E. & Griffiths, K. The effect of prolactin on human BPH epithelial cell proliferation. Prostate 6, 145–153 (1985).
Leav, I. et al. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am. J. Pathol. 154, 863–870 (1999).
Rannikko, S. & Adlercreutz, H. Plasma estradiol, free testosterone, sex hormone binding globulin binding capacity, and prolactin in benign prostatic hyperplasia and prostatic cancer. Prostate 4, 223–229 (1983).
Colao, A. et al. Prolactin and prostate hypertrophy: a pilot observational, prospective, case-control study in men with prolactinoma. J. Clin. Endocrinol. Metab. 89, 2770–2775 (2004).
Nickel, J. C. Inflammation and benign prostatic hyperplasia. Urol. Clin. North Am. 35, 109–115 (2008).
Wang, L., Yang, J. R., Yang, L. Y. & Liu, Z. T. Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med. Hypotheses 70, 1021–1023 (2008).
De Marzo, A. M. et al. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7, 256–269 (2007).
Tangbanluekal, L. & Robinette, C. L. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology 132, 2407–2416 (1993).
Gilleran, J. P. et al. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology 144, 2046–2054 (2003).
McPherson, S. J. et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology 142, 2458–2467 (2001).
Yatkin, E., Bernoulli, J., Talvitie, E. M. & Santti, R. Inflammation and epithelial alterations in rat prostate: impact of the androgen to oestrogen ratio. Int. J. Androl. 32, 399–410 (2009).
Stattin, P. et al. Plasma prolactin and prostate cancer risk: A prospective study. Int. J. Cancer 92, 463–465 (2001).
Gann, P. H., Hennekens, C. H., Ma, J., Longcope, C. & Stampfer, M. J. Prospective study of sex hormone levels and risk of prostate cancer. J. Natl Cancer Inst. 88, 1118–1126 (1996).
Eaton, N. E., Reeves, G. K., Appleby, P. N. & Key, T. J. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br. J. Cancer 80, 930–934 (1999).
Melck, D. et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 141, 118–126 (2000).
Janssen, T. et al. In vitro characterization of prolactin-induced effects on proliferation in the neoplastic LNCaP, DU145, and PC3 models of the human prostate. Cancer 77, 144–149 (1996).
Xu, X., Kreye, E., Kuo, C. B. & Walker, A. M. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when du145 human prostate cancer cells were grown in nude mice. Cancer Res. 61, 6098–6104 (2001).
Peirce, S. K., Chen, W. Y. & Chen, W. Y. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J. Endocrinol. 171, R1–R4 (2001).
Ahonen, T. J. et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J. Biol. Chem. 278, 27287–27292 (2003).
Suwa, T., Nyska, A., Haseman, J. K., Mahler, J. F. & Maronpot, R. R. Spontaneous lesions in control B6C3F1 mice and recommended sectioning of male accessory sex organs. Toxicol. Pathol. 30, 228–234 (2002).
Lawson, D. A. et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc. Natl Acad. Sci. USA 107, 2610–2615.
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Rao, Y. P., Buckley, D. J. & Buckley, A. R. Rapid activation of mitogen-activated protein kinase and p21ras by prolactin and interleukin 2 in rat Nb2 node lymphoma cells. Cell Growth Differ. 6, 1235–1244 (1995).
Erwin, R. A., Kirken, R. A., Malabarba, M. G., Farrar, W. L. & Rui, H. Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology 136, 3512–3518 (1995).
Buckley, A. R., Rao, Y. P., Buckley, D. J. & Gout, P. W. Prolactin-induced phosphorylation and nuclear translocation of MAP kinase in Nb2 lymphoma cells. Biochem. Biophys. Res. Commun. 204, 1158–1164 (1994).
Clevenger, C. V. & Medaglia, M. V. The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol. Endocrinol. 8, 674–681 (1994).
Berlanga, J. J., Vara, J. A. F., Martin-Perez, J. & Garcia-Ruiz, J. P. Prolactin receptor is associated with c-src kinase in rat liver. Mol. Endocrinol. 9, 1461–1467 (1995).
Too, C. K., Shiu, R. P. & Friesen, H. G. Cross-linking of G.-proteins to the prolactin receptor in rat NB2 lymphoma cells. Biochem. Biophys. Res. Commun. 173, 48–52 (1990).
Berlanga, J. J. et al. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J. Biol. Chem. 272, 2050–2052 (1997).
al-Sakkaf, K. A., Dobson, P. R. & Brown, B. L. Activation of phosphatidylinositol 3-kinase by prolactin in Nb2 cells. Biochem. Biophys. Res. Commun. 221, 779–784 (1996).
Clevenger, C. V., Furth, P. A., Hankinson, S. E. & Schuler, L. A. The role of prolactin in mammary carcinoma. Endocr. Rev. 24, 1–27 (2003).
Neilson, L. M. et al. Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol. Endocrinol. 21, 2218–2232 (2007).
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Yu, H. & Jove, R. The STATs of cancer—new molecular targets come of age. Nat. Rev. Cancer 4, 97–105 (2004).
Schindler, C., Levy, D. E. & Decker, T. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 282, 20059–20063 (2007).
Mui, A. et al. Function of the common beta subunit of the GM-CSF/IL-3/IL-5 receptors. Adv. Exp. Med. Biol. 365, 217–223 (1994).
Liu, X., Robinson, G. W., Gouilleux, F., Groner, B. & Hennighausen, L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl Acad. Sci. USA 92, 8831–8835 (1995).
Liu, J. X., Mietz, J., Modi, W. S., John, S. & Leonard, W. J. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J. Biol. Chem. 271, 10738–10744 (1996).
Reich, N. C. & Liu, L. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 6, 602–612 (2006).
Vinkemeier, U. Getting the message across, STAT! Design principles of a molecular signaling circuit. J. Cell Biol. 167, 197–201 (2004).
Sekimoto, T., Nakajima, K., Tachibana, T., Hirano, T. & Yoneda, Y. Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J. Biol. Chem. 271, 31017–31020 (1996).
Sekimoto, T., Imamoto, N., Nakajima, K., Hirano, T. & Yoneda, Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 16, 7067–7077 (1997).
Kawashima, T. et al. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol. Cell Biol. 29, 1796–1813 (2009).
Marg, A. et al. Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. J. Cell Biol. 165, 823–833 (2004).
Soldaini, E. et al. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol. Cell Biol. 20, 389–401 (2000).
Dagvadorj, A., Kirken, R. A., Leiby, B., Karras, J. & Nevalainen, M. T. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin. Cancer Res. 14, 1317–1324 (2008).
Li, H. et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin. Cancer Res. 11, 5863–5868 (2005).
Kazansky, A. V., Spencer, D. M. & Greenberg, N. M. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res. 63, 8757–8762 (2003).
Gu, L. et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am. J. Pathol. 176, 1959–1972 (2010).
Gu, L. et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr. Relat. Cancer 17, 481–493 (2010).
Tan, S. H. et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 68, 236–248 (2008).
Isaacs, J. T. & Isaacs, W. B. Androgen receptor outwits prostate cancer drugs. Nat. Med. 10, 26–27 (2004).
Thomas, C. et al. Transcription factor Stat5 knockdown enhances androgen receptor degradation and delays castration-resistant prostate cancer progression in vivo. Mol. Cancer Ther. 10, 347–359.
Feldman, B. J. & Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1, 34–45 (2001).
Goffin, V., Bernichtein, S., Touraine, P. & Kelly, P. A. Development and potential clinical uses of human prolactin receptor antagonists. Endocr. Rev. 26, 400–422 (2005).
Gillam, M. P., Molitch, M. E., Lombardi, G. & Colao, A. Advances in the treatment of prolactinomas. Endocr. Rev. 27, 485–534 (2006).
Arrighi, N. et al. Molecular and pharmacological detection of dopaminergic receptors in the human male urinary tract. Neurourol. Urodyn. 28, 343–348 (2009).
Arvigo, M. et al. Somatostatin and dopamine receptor interaction in prostate and lung cancer cell lines. J. Endocrinol. 207, 309–317 (2010).
Eyal, O., Jomain, J. B., Kessler, C., Goffin, V. & Handwerger, S. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol. Reprod. 76, 777–783 (2007).
Howell, S. J., Anderson, E., Hunter, T., Farnie, G. & Clarke, R. B. Prolactin receptor antagonism reduces the clonogenic capacity of breast cancer cells and potentiates doxorubicin and paclitaxel cytotoxicity. Breast Cancer Res. 10, R68 (2008).
Llovera, M. et al. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene 19, 4695–4705 (2000).
Tallet, E. et al. Rational design of competitive prolactin/growth hormone receptor antagonists. J. Mammary Gland Biol. Neoplasia 13, 105–117 (2008).
Wu, W., Ginsburg, E., Vonderhaar, B. K. & Walker, A. M. S179D prolactin increases vitamin D receptor and p21 through up-regulation of short 1b prolactin receptor in human prostate cancer cells. Cancer Res. 65, 7509–7515 (2005).
Xu, J. et al. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol. Endocrinol. 25, 597–610 (2011).
Tefferi, A. & Gilliland, D. G. The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin. Proc. 80, 947–958 (2005).
Levine, R. L. et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106, 3377–3379 (2005).
Frohling, S. et al. Rare occurrence of the JAK2 V617F mutation in AML subtypes M5, M6, and M7. Blood 107, 1242–1243 (2006).
Gu, L. et al. Activating mutation (V617F) in the tyrosine kinase JAK2 is absent in locally-confined or castration-resistant prostate cancer. Anal. Cell Pathol. (Amst). 33, 55–59 (2010).
Ferrajoli, A. et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 67, 11291–11299 (2007).
Hedvat, M. et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16, 487–497 (2009).
Guh, J. Y. et al. Advanced glycation end product-induced proliferation in NRK-49F cells is dependent on the JAK2/STAT5 pathway and cyclin D1. Am. J. Kidney Dis. 38, 1096–1104 (2001).
Nagel-Wolfrum, K. et al. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol. Cancer Res. 2, 170–182 (2004).
Turkson, J. et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 276, 45443–45455 (2001).
Turkson, J. et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol. Cancer Ther. 3, 261–269 (2004).
Numata, A. et al. Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway. J. Biol. Chem. 280, 12621–12629 (2005).
Malin, S. et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol. 11, 171–179 (2010).
Goffin, V. & Kelly, P. A. The prolactin/growth hormone receptor family: structure/function relationships. J. Mammary Gland Biol. Neoplasia 2, 7–17 (1997).
Hu, Z. Z., Meng, J. & Dufau, M. L. Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J. Biol. Chem. 276, 41086–41094 (2001).
Arden, K. C., Boutin, J. M., Djiane, J., Kelly, P. A. & Cavenee, W. K. The receptors for prolactin and growth hormone are localized in the same region of human chromosome 5. Cytogenet. Cell Genet. 53, 161–165 (1990).
Kelly, P. A. et al. The growth hormone/prolactin receptor family. Recent Prog. Horm. Res. 48, 123–164 (1993).
Boutin, J. M. et al. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell 53, 69–77 (1988).
Buteau, H. et al. N-glycosylation of the prolactin receptor is not required for activation of gene transcription but is crucial for its cell surface targeting. Mol. Endocrinol. 12, 544–55 (1998).
Rui, H., Kirken, R. A. & Farrar, W. L. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J. Biol. Chem. 269, 5364–5368 (1994).
Becker, S., Groner, B. & Muller, C. W. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature 394, 145–151 (1998).
Chen, X. et al. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93, 827–839 (1998).
Decker, T. & Kovarik, P. Serine phosphorylation of STATs. Oncogene 19, 2628–2637 (2000).
Decker, T. & Kovarik, P. Transcription factor activity of STAT proteins: structural requirements and regulation by phosphorylation and interacting proteins. Cell. Mol. Life Sci. 55, 1535–1546 (1999).
Horvath, C. M., Wen, Z. & Darnell, J. E. Jr. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 9, 984–994 (1995).
Acknowledgements
The authors are grateful to Dr Lucila Sackmann-Sala for critical reading of this manuscript, and to Dr Nira Ben-Jonathan for helpful discussions during the revision of this manuscript. The authors are supported by grants from the Association for International Cancer Research (AICR Grant 05-0603) and NIH National Cancer Institute (Grant 1RO1CA113580-01A1).
Author information
Authors and Affiliations
Contributions
V. Goffin, D. T. Hoang, and M. T. Nevalainen researched data for the article, made substantial contributions to discussions of content, wrote the article and reviewed and edited the article before submission. R. L. Bogorad researched data for the article and reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goffin, V., Hoang, D., Bogorad, R. et al. Prolactin regulation of the prostate gland: a female player in a male game. Nat Rev Urol 8, 597–607 (2011). https://doi.org/10.1038/nrurol.2011.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.143
This article is cited by
-
Prolactin receptor signaling induces acquisition of chemoresistance and reduces clonogenicity in acute myeloid leukemia
Cancer Cell International (2023)
-
Local gingival crevicular fluid, synovial fluid, and circulating levels of prolactin hormone in patients with moderately active rheumatoid arthritis and stage III and IV periodontitis before and after non-surgical periodontal treatment—a controlled trial
Clinical Oral Investigations (2023)
-
Prostate luminal progenitor cells: from mouse to human, from health to disease
Nature Reviews Urology (2022)
-
ABBV-176, a PRLR antibody drug conjugate with a potent DNA-damaging PBD cytotoxin and enhanced activity with PARP inhibition
BMC Cancer (2021)
-
Seasonal Expression of Prolactin Receptor in the Scented Gland of Male Muskrat (Ondatra zibethicus)
Scientific Reports (2015)