Abstract
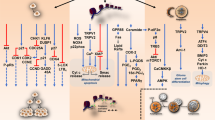
Cannabinoids, their receptors and their metabolizing enzymes are emerging as a new regulatory system, which is involved in multiple physiological functions. Normal prostate tissue expresses several constituents of the endocannabinoid system including the CB1 receptor, receptors belonging to the transient receptor potential family and fatty acid amide hydrolase, a hydrolyzing enzyme, all of which have been localized in the glandular epithelia. Accumulating evidence indicate that the endocannabinoid system is dysregulated in prostate cancer, suggesting that it has a role in prostate homeostasis. Overexpression of several components of the endocannabinoid system correlate with prostate cancer grade and progression, potentially providing a new therapeutic target for prostate cancer. Moreover, several cannabinoids exert antitumoral properties against prostate cancer, reducing xenograft prostate tumor growth, prostate cancer cell proliferation and cell migration. Although the therapeutic potential of cannabinoids against prostate cancer is very promising, future research using animal models is needed to evaluate the influence of systemic networks in their antitumoral action.
Key Points
-
The prostate gland expresses several components of the endocannabinoid system, including the CB1 receptor, TRPV1 receptor and the hydrolyzing enzyme fatty acid amide hydrolase
-
Many of the components of the endocannabinoid system are dysregulated in prostate cancer, and their levels correlate with malignant grade, suggesting that they could be novel prognostic markers
-
Several cannabinoids, including WIN-55,212-2, THC, methanandamide and the CB2 agonist JWH-015, exert antitumoral effects against prostate cancer in vivo and in cultured prostate cancer cell lines
-
Cannabinoids induce cell cycle arrest and apoptosis through ceramide generation and Akt inhibition in cells in vitro
-
Studies have suggested a role for the cannabinoid receptor CB2 in the antiproliferative effect of cannabinoids on prostate cancer cells
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C. & Bonner, T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 (1990).
Kogan, N. M. & Mechoulam, R. Cannabinoids in health and disease. Dialogues Clin. Neurosci. 9, 413–430 (2007).
Pertwee, R. G. et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol. Rev. 62, 588–631 (2010).
Guindon, J. & Hohmann, A. G. The endocannabinoid system and cancer: therapeutic implications. Br. J. Pharmacol. 163, 1447–1463 (2011).
Fowler, C. J. et al. Targeting the endocannabinoid system for the treatment of cancer—a practical view. Curr. Top. Med. Chem. 10, 814–827 (2010).
Oesch, S. & Gertsch, J. Cannabinoid receptor ligands as potential anticancer agents—high hopes for new therapies? J. Pharm. Pharmacol. 61, 839–853 (2009).
Alexander, A., Smith, P. F. & Rosengren, R. J. Cannabinoids in the treatment of cancer. Cancer Lett. 285, 6–12 (2009).
Velasco, G. et al. Cannabinoids and gliomas. Mol. Neurobiol. 36, 60–67 (2007).
Vara, D. et al. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 18, 1099–1111 (2011).
Alger, B. E. & Kim, J. Supply and demand for endocannabinoids. Trends Neurosci. 34, 304–315 (2011).
Hanus, L. et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl Acad. Sci. USA 98, 3662–3665 (2001).
Oka, S. et al. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J. Neurochem. 85, 1374–1381 (2003).
Brown, I. et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 31, 1584–1591 (2010).
Tan, B. et al. Targeted lipidomics: discovery of new fatty acyl amides. AAPS J. 8, E461–E465 (2006).
Wang, J. & Ueda, N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 89, 112–119 (2009).
Ueda, N., Tsuboi, K., Uyama, T. & Ohnishi, T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors 37, 1–7 (2011).
Savinainen, J. R., Saario, S. M. & Laitinen, J. T. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol. (Oxf.) http://dx.doi.org/10.1111/j.1748-17162011.02280.x.
Stark, K., Dostalek, M. & Guengerich, F. P. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS J. 275, 3706–3717 (2008).
Diaz-Laviada, I. & Ruiz-Llorente, L. Signal transduction activated by cannabinoid receptors. Mini Rev. Med. Chem. 5, 619–630 (2005).
Rozenfeld, R. & Devi, L. A. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 22, 2311–2322 (2008).
Patel, K. D., Davison, J. S., Pittman, Q. J. & Sharkey, K. A. Cannabinoid CB(2) receptors in health and disease. Curr. Med. Chem. 17, 1393–1410 (2010).
Atwood, B. K. & Mackie, K. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160, 467–479 (2010).
Morgan, N. H., Stanford, I. M. & Woodhall, G. L. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 57, 356–368 (2009).
Pertwee, R. G. GPR55: a new member of the cannabinoid receptor clan? Br. J. Pharmacol. 152, 984–986 (2007).
Ross, R. A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 30, 156–163 (2009).
Okuno, T. & Yokomizo, T. What is the natural ligand of GPR55? J. Biochem. 149, 495–497 (2011).
Schroder, F. H. Prostate cancer around the world. An overview. Urol. Oncol. 28, 663–667 (2010).
Quon, H., Loblaw, A. & Nam, R. Dramatic increase in prostate cancer cases by 2021. BJU Int. http://dx.doi.org/10.1111/j.1464-410X.2011.10197.x.
Webber, M. M., Bello, D. & Quader, S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications Part 2. Tumorigenic cell lines. Prostate 30, 58–64 (1997).
Gratzke, C. et al. Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: relation to nerves and interstitial cells. Eur. Urol. 57, 902–910 (2010).
Tokanovic, S., Malone, D. T. & Ventura, S. Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland. Br. J. Pharmacol. 150, 227–234 (2007).
Chung, S. C. et al. A high cannabinoid CB(1) receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 45, 174–182 (2009).
Ruiz-Llorente, L. et al. Expression of functionally active cannabinoid receptor CB1 in the human prostate gland. Prostate 54, 95–102 (2003).
Czifra, G. et al. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 135, 507–514 (2009).
Dhanasekaran, S. M. et al. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. FASEB J. 19, 243–245 (2005).
Wang, J. et al. Expression and secretion of N.-acylethanolamine-hydrolysing acid amidase in human prostate cancer cells. J. Biochem. 144, 685–690 (2008).
Nithipatikom, K. et al. 2-arachidonoylglycerol: a novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 64, 8826–8830 (2004).
Endsley, M. P. et al. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: location, hydrolysis and 12-lipoxygenase metabolism. Int. J. Cancer 121, 984–991 (2007).
Schuel, H. et al. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 121, 211–227 (2002).
Sarfaraz, S., Afaq, F., Adhami, V. M. & Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 65, 1635–1641 (2005).
Thors, L. et al. Fatty acid amide hydrolase in prostate cancer: association with disease severity and outcome, CB1 receptor expression and regulation by IL-4. PLoS ONE 5, e12275 (2010).
Fowler, C. J., Hammarsten, P. & Bergh, A. Tumour Cannabinoid CB(1) receptor and phosphorylated epidermal growth factor receptor expression are additive prognostic markers for prostate cancer. PLoS ONE 5, e15205 (2010).
Mimeault, M., Pommery, N., Wattez, N., Bailly, C. & Henichart, J. P. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate 56, 1–12 (2003).
Sanchez, M. G., Ruiz-Llorente, L., Sanchez, A. M. & Diaz-Laviada, I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell. Signal. 15, 851–859 (2003).
Olea-Herrero, N., Vara, D., Malagarie-Cazenave, S. & Diaz-Laviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2. Br. J. Cancer 101, 940–950 (2009).
Pineiro, R., Maffucci, T. & Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30, 142–152 (2011).
Andradas, C. et al. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30, 245–252 (2011).
Ross, R. A. L-alpha-Lysophosphatidylinositol meets GPR55: a deadly relationship. Trends Pharmacol. Sci. 32, 265–269 (2011).
Balenga, N. A. et al. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. http://dx.doi.org/10.1038/cr.2011.60.
Endsley, M. P. et al. Expression and function of fatty acid amide hydrolase in prostate cancer. Int. J. Cancer 123, 1318–1326 (2008).
Sarfaraz, S., Afaq, F., Adhami, V. M., Malik, A. & Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 281, 39480–39491 (2006).
Sanchez, M. G., Sanchez, A. M., Ruiz-Llorente, L. & Diaz-Laviada, I. Enhancement of androgen receptor expression induced by (R)-methanandamide in prostate LNCaP cells. FEBS Lett. 555, 561–566 (2003).
Cudaback, E., Marrs, W., Moeller, T. & Stella, N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS ONE 5, e8702 (2010).
Nithipatikom, K., Isbell, M. A., Endsley, M. P., Woodliff, J. E. & Campbell, W. B. Anti-proliferative effect of a putative endocannabinoid, 2-arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat. 94, 34–43 (2011).
Ligresti, A. et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 318, 1375–1387 (2006).
Ruiz, L., Miguel, A. & Diaz-Laviada, I. Delta9-tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 458, 400–404 (1999).
Melck, D. et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 141, 118–126 (2000).
Sakaki, K. & Kaufman, R. J. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy 4, 841–843 (2008).
Ponnusamy, S. et al. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 6, 1603–1624 (2010).
He, C. & Klionsky, D. J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 (2009).
Qin, L., Wang, Z., Tao, L. & Wang, Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6, 239–247 (2010).
Salazar, M. et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 (2009).
Diaz-Laviada, I. Effect of capsaicin on prostate cancer cells. Future Oncol. 6, 1545–1550 (2010).
Bradford, P. G. & Awad, A. B. Modulation of signal transduction in cancer cells by phytosterols. Biofactors 36, 241–247 (2010).
Liu, X. et al. Acid ceramidase upregulation in prostate cancer: role in tumor development and implications for therapy. Expert Opin. Ther. Targets 13, 1449–1458 (2009).
Turner, L. S. et al. Autophagy is increased in prostate cancer cells overexpressing acid ceramidase and enhances resistance to C6 ceramide. Prostate Cancer Prostatic Dis. 14, 30–37 (2011).
Kuc, C., Jenkins, A. & Van Dross, R. T. Arachidonoyl ethanolamide (AEA)-induced apoptosis is mediated by J-series prostaglandins and is enhanced by fatty acid amide hydrolase (FAAH) blockade. Mol. Carcinog. http://dx.doi.org/10.1002/mc.20770.
Olea-Herrero, N., Vara, D., Malagarie-Cazenave, S. & Diaz-Laviada, I. The cannabinoid R+ methanandamide induces IL-6 secretion by prostate cancer PC3 cells. J. Immunotoxicol. 6, 249–256 (2009).
Xu, M. et al. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin. Dev. Immunol. http://dx.doi.org/10.1155/2010/832454.
Wilke, C. M. et al. Th17 cells in cancer: help or hindrance? Carcinogenesis 32, 643–649 (2011).
Zou, W. & Restifo, N. P. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10, 248–256 (2010).
O'Sullivan, S. E. & Kendall, D. A. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology 215, 611–616 (2010).
Wang, Y. X. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 20, 124–137 (2010).
Sun, Y. & Bennett, A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007, 23513 (2007).
Youssef, J. & Badr, M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br. J. Pharmacol. 164, 68–82 (2011).
Nakamura, Y., Suzuki, T., Sugawara, A., Arai, Y. & Sasano, H. Peroxisome proliferator-activated receptor gamma in human prostate carcinoma. Pathol. Int. 59, 288–293 (2009).
Hisatake, J. I. et al. Down-regulation of prostate-specific antigen expression by ligands for peroxisome proliferator-activated receptor gamma in human prostate cancer. Cancer Res. 60, 5494–5498 (2000).
Ikezoe, T. et al. Mutational analysis of the peroxisome proliferator-activated receptor gamma gene in human malignancies. Cancer Res. 61, 5307–5310 (2001).
Kubota, T. et al. Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 58, 3344–3352 (1998).
Matsuyama, M. & Yoshimura, R. The target of arachidonic acid pathway is a new anticancer strategy for human prostate cancer. Biologics 2, 725–732 (2008).
Nagata, D. et al. Peroxisome proliferator-activated receptor-gamma and growth inhibition by its ligands in prostate cancer. Cancer Detect Prev. 32, 259–266 (2008).
Jiang, M. et al. Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy. Cell Death Differ. 17, 469–481 (2010).
Pan, Z., Yang, H. & Reinach, P. S. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genomics 5, 108–116 (2011).
De Petrocellis, L. et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494 (2010).
Akopian, A. N., Ruparel, N. B., Jeske, N. A., Patwardhan, A. & Hargreaves, K. M. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol. Sci. 30, 79–84 (2009).
Van Haute, C., De Ridder, D. & Nilius, B. TRP channels in human prostate. Scientific World Journal 10, 1597–1611 (2010).
Wang, H. P., Pu, X. Y. & Wang, X. H. Distribution profiles of transient receptor potential melastatin-related and vanilloid-related channels in prostatic tissue in rat. Asian J. Androl. 9, 634–640 (2007).
Prevarskaya, N., Skryma, R., Bidaux, G., Flourakis, M. & Shuba, Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 14, 1295–1304 (2007).
Du, S. et al. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology 72, 450–455 (2008).
Van der Aa, F., Roskams, T., Blyweert, W. & De Ridder, D. Interstitial cells in the human prostate: a new therapeutic target? Prostate 56, 250–255 (2003).
Sanchez, M. G. et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 515, 20–27 (2005).
Ziglioli, F. et al. Vanilloid-mediated apoptosis in prostate cancer cells through a TRPV-1 dependent and a TRPV-1-independent mechanism. Acta Biomed. 80, 13–20 (2009).
Maccarrone, M., Lorenzon, T., Bari, M., Melino, G. & Finazzi-Agro, A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 275, 31938–31945 (2000).
Guilak, F., Leddy, H. A. & Liedtke, W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann. N. Y. Acad. Sci. 1192, 404–409 (2010).
Watanabe, H. et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 (2003).
Cohen, D. M. TRPV4 and the mammalian kidney. Pflugers Arch. 451, 168–175 (2005).
Tsavaler, L., Shapero, M. H., Morkowski, S. & Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769 (2001).
Thebault, S. et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 280, 39423–39435 (2005).
Bai, V. U. et al. Androgen regulated TRPM8 expression: a potential mRNA marker for metastatic prostate cancer detection in body fluids. Int. J. Oncol. 36, 443–450 (2010).
Zhang, L. & Barritt, G. J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 64, 8365–8373 (2004).
Romanuik, T. L. et al. LNCaP Atlas: gene expression associated with in vivo progression to castration-recurrent prostate cancer. BMC Med. Genomics 3, 43 (2010).
De Petrocellis, L. et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 325, 1007–1015 (2008).
Valero, M., Morenilla-Palao, C., Belmonte, C. & Viana, F. Pharmacological and functional properties of TRPM8 channels in prostate tumor cells. Pflugers Arch. 461, 99–114 (2011).
Kulkarni, P. TRPM8 and prostate cancer: to overexpress or repress, that is the question—comment on “Effects of TRPM8 on proliferation and motility of prostate cancer PC-3 cells” by Yang, Z. H. et al. in Asian Journal of Andrology. Asian J. Androl. 11, 150–151 (2009).
Yang, Z. H., Wang, X. H., Wang, H. P. & Hu, L. Q. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J. Androl. 11, 157–165 (2009).
Acknowledgements
This work was supported by Ministerio de Ciencia e Innovación (grant SAF2008-03220), Comunidad de Madrid (grants CCG10-UAH/SAL-5956 and CAM S-SAL-0261-2006), Comunidad Castilla-LaMancha (Grant POII11-0159-0054).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Díaz-Laviada, I. The endocannabinoid system in prostate cancer. Nat Rev Urol 8, 553–561 (2011). https://doi.org/10.1038/nrurol.2011.130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.130
This article is cited by
-
Metabolomic profile of prostate cancer-specific survival among 1812 Finnish men
BMC Medicine (2022)
-
Effects of orthotopic implantation of rat prostate tumour cells upon components of the N-acylethanolamine and monoacylglycerol signalling systems: an mRNA study
Scientific Reports (2020)
-
Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis
Journal of Physiology and Biochemistry (2018)
-
The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells
Prostate Cancer and Prostatic Diseases (2016)