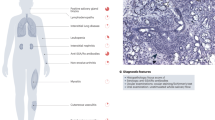
Key Points
-
Genetic risk factors associated with primary Sjögren syndrome (pSS) can promote B cell activation, as can epigenetic changes, which can sometimes affect the same disease-associated genes.
-
BAFF (also known as TNF ligand superfamily member 13B) is central to the crosstalk between early activation of the innate immune system and the stimulation of autoreactive B cells.
-
CD27+ memory B cells, marginal zone B cells, plasmablasts and plasma cells are the key subsets of B cells involved in the pathogenesis of pSS.
-
Target organs (salivary and lachrymal glands) are involved in B cell activation, notably via the formation of germinal centre-like structures within the epithelium and plasma cell niches.
-
Continuous stimulation of autoreactive B cells by immune complexes is the first step towards lymphomagenesis associated with pSS.
-
Continuously activated autoreactive B cells need to be tightly controlled by genetic factors and by the tissue microenvironment as subtle defects in these controls might promote clonal escape and lymphomagenesis.
Abstract
Primary Sjögren syndrome (pSS) is a prototypical autoimmune disease. The involvement of B cells in the pathogenesis of pSS has long been suspected on the basis of clinical observations that include the presence of serum autoantibodies, hypergammaglobulinaemia, increased levels of free light chains and increased risk of B cell lymphoma. Moreover, the composition of the B cell subset is altered in pSS. In this Review, we discuss the mechanisms that support the increased activation of B cells in pSS, including genetic and epigenetic factors and environmental triggers that promote B cell activation via the innate immune system. B cell activating factor (BAFF, also known as TNF ligand superfamily member 13B) is at the crossroads of this process. An important role also exists for the target tissue (exocrine glands, namely the salivary and lachrymal glands), which promotes local B cell activation. This continuous stimulation of B cells is the main driver of lymphomatous escape. Identification of the multiple steps that support B cell activation has led to the development of promising targeted therapies that will hopefully lead to the development of an efficient therapeutic strategy for pSS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Qin, B. et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann. Rheum. Dis. 74, 1983–1989 (2015).
Bowman, S. J., Ibrahim, G. H., Holmes, G., Hamburger, J. & Ainsworth, J. R. Estimating the prevalence among Caucasian women of primary Sjögren's syndrome in two general practices in Birmingham, U.K. Scand. J. Rheumatol. 33, 39–43 (2004).
Gottenberg, J.-E. et al. Serum levels of β2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren's syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS ONE 8, e59868 (2013).
Jonsson, R., Theander, E., Sjöström, B., Brokstad, K. & Henriksson, G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 310, 1854–1855 (2013).
Theander, E. et al. Prediction of Sjögren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 67, 2427–2436 (2015).
Risselada, A. P., Looije, M. F., Kruize, A. A., Bijlsma, J. W. J. & van Roon, J. A. G. The role of ectopic germinal centers in the immunopathology of primary Sjögren's syndrome: a systematic review. Semin. Arthritis Rheum. 42, 368–376 (2013).
Zintzaras, E., Voulgarelis, M. & Moutsopoulos, H. M. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch. Intern. Med. 165, 2337–2344 (2005).
Nocturne, G. et al. Rheumatoid factor and disease activity are independent predictors of lymphoma in primary Sjögren's Syndrome. Arthritis Rheumatol. 68, 977–985 (2016).
Nocturne, G. & Mariette, X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat. Rev. Rheumatol. 9, 544–556 (2013).
Lessard, C. J. et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat. Genet. 45, 1284–1292 (2013).
Li, Y. et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren's syndrome at 7q11.23. Nat. Genet. 45, 1361–1365 (2013).
Nocturne, G. et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjögren's syndrome. Blood 122, 4068–4076 (2013).
Nocturne, G. et al. Germline variation of TNFAIP3 in primary Sjögren's syndrome-associated lymphoma. Ann. Rheum. Dis. 75, 780–783 (2016).
Taylor, K. E. et al. Genome-wide association analysis reveals genetic heterogeneity of Sjögren's syndrome according to ancestry. Arthritis Rheumatol. 69, 1294–1305 (2017).
Altorok, N. et al. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary Sjögren's syndrome. Arthritis Rheumatol. 66, 731–739 (2014).
Miceli-Richard, C. et al. Overlap between differentially methylated DNA regions in blood B lymphocytes and genetic at-risk loci in primary Sjögren's syndrome. Ann. Rheum. Dis. 75, 933–940 (2016).
Imgenberg-Kreuz, J. et al. Genome-wide DNA methylation analysis in multiple tissues in primary Sjögren's syndrome reveals regulatory effects at interferon-induced genes. Ann. Rheum. Dis. 75, 2029–2036 (2016).
Konsta, O. D. et al. Defective DNA methylation in salivary gland epithelial acini from patients with Sjögren's syndrome is associated with SSB gene expression, anti-SSB/La detection, and lymphocyte infiltration. J. Autoimmun. 68, 30–38 (2016).
Cole, M. B. et al. Epigenetic signatures of salivary gland inflammation in Sjögren's syndrome. Arthritis Rheumatol. 68, 2936–2944 (2016).
Thabet, Y. et al. Epigenetic dysregulation in salivary glands from patients with primary Sjögren's syndrome may be ascribed to infiltrating B cells. J. Autoimmun. 41, 175–181 (2013).
Peng, L. et al. MicroRNA profiling in Chinese patients with primary Sjögren syndrome reveals elevated miRNA-181a in peripheral blood mononuclear cells. J. Rheumatol. 41, 2208–2213 (2014).
Shi, H., Zheng, L., Zhang, P. & Yu, C. miR-146a and miR-155 expression in PBMCs from patients with Sjögren's syndrome. J. Oral Pathol. Med. 43, 792–797 (2014).
Pauley, K. M. et al. Altered miR-146a expression in Sjögren's syndrome and its functional role in innate immunity. Eur. J. Immunol. 41, 2029–2039 (2011).
Alevizos, I., Alexander, S., Turner, R. J. & Illei, G. G. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren's syndrome. Arthritis Rheum. 63, 535–544 (2011).
Wang-Renault, S.-F. et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjögren's syndrome. Ann. Rheum. Dis. 77, 133–140 (2018).
Pender, M. P. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 24, 584–588 (2003).
Borza, C. M. & Hutt-Fletcher, L. M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8, 594–599 (2002).
Harley, J. B. & Zoller, E. E. What caused all these troubles, anyway? Epstein-Barr virus in Sjögren's syndrome reevaluated. Arthritis Rheumatol. 66, 2328–2330 (2014).
Fleck, M., Kern, E. R., Zhou, T., Lang, B. & Mountz, J. D. Murine cytomegalovirus induces a Sjögren's syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheum. 41, 2175–2184 (1998).
Iwakiri, D. et al. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 206, 2091–2099 (2009).
Küppers, R. B cells under influence: transformation of B cells by Epstein–Barr virus. Nat. Rev. Immunol. 3, 801–812 (2003).
He, B., Raab-Traub, N., Casali, P. & Cerutti, A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J. Immunol. 171, 5215–5224 (2003).
Inoue, H. et al. Possible involvement of EBV-mediated α-fodrin cleavage for organ-specific autoantigen in Sjögren's syndrome. J. Immunol. 166, 5801–5809 (2001).
Croia, C. et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjögren's syndrome. Arthritis Rheumatol. 66, 2545–2557 (2014).
Minamitani, T. et al. Evasion of affinity-based selection in germinal centers by Epstein-Barr virus LMP2A. Proc. Natl Acad. Sci. USA 112, 11612–11617 (2015).
Brauner, S. et al. H1N1 vaccination in Sjögren's syndrome triggers polyclonal B cell activation and promotes autoantibody production. Ann. Rheum. Dis. 76, 1755–1763 (2017).
Volkman, H. E. & Stetson, D. B. The enemy within: endogenous retroelements and autoimmune disease. Nat. Immunol. 15, 415–422 (2014).
Zeng, M. et al. MAVS, cGAS, and endogenous retroviruses in T-independent B cell responses. Science 346, 1486–1492 (2014).
Mavragani, C. P. et al. Expression of long interspersed nuclear element 1 retroelements and induction of type I interferon in patients with systemic autoimmune disease. Arthritis Rheumatol. 68, 2686–2696 (2016).
Crow, M. K. Long interspersed nuclear elements (LINE-1): potential triggers of systemic autoimmune disease. Autoimmunity 43, 7–16 (2010).
Hung, T. et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 350, 455–459 (2015).
Bohnhorst, J. Ø., Bjørgan, M. B., Thoen, J. E., Natvig, J. B. & Thompson, K. M. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren's syndrome. J. Immunol. 167, 3610–3618 (2001).
d'Arbonneau, F. et al. BAFF-induced changes in B cell antigen receptor-containing lipid rafts in Sjögren's syndrome. Arthritis Rheum. 54, 115–126 (2006).
Corneth, O. B. J. et al. Enhanced Bruton's tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheumatol. 69, 1313–1324 (2017).
Hansen, A. et al. Abnormalities in peripheral B cell memory of patients with primary Sjögren's syndrome. Arthritis Rheum. 50, 1897–1908 (2004).
Roberts, M. E. P. et al. Primary Sjögren's syndrome is characterized by distinct phenotypic and transcriptional profiles of IgD+ unswitched memory B cells. Arthritis Rheumatol. 66, 2558–2569 (2014).
Szabó, K., Papp, G., Szántó, A., Tarr, T. & Zeher, M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren's syndrome and systemic lupus erythematosus. Clin. Exp. Immunol. 183, 76–89 (2016).
Hamza, N., Bos, N. A. & Kallenberg, C. G. M. B-Cell populations and sub-populations in Sjögren's syndrome. Presse Med. 41, e475–483 (2012).
Hansen, A. et al. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 46, 2160–2171 (2002).
Mingueneau, M. et al. Cytometry by time-of-flight immunophenotyping identifies a blood Sjögren's signature correlating with disease activity and glandular inflammation. J. Allergy Clin. Immunol. 137, 1809–1821.e12 (2016).
Halliley, J. L. et al. Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity 43, 132–145 (2015).
Mei, H. E. et al. Plasmablasts with a mucosal phenotype contribute to plasmacytosis in SLE. Arthritis Rheumatol. 69, 2018–2018 (2017).
Jasiek, M. et al. A multicentre study of 95 biopsy-proven cases of renal disease in primary Sjögren's syndrome. Rheumatology (Oxford) 56, 362–370 (2017).
Tengnér, P., Halse, A. K., Haga, H. J., Jonsson, R. & Wahren-Herlenius, M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren's syndrome. Arthritis Rheum. 41, 2238–2248 (1998).
Fletcher, C. A. et al. Development of nephritis but not sialadenitis in autoimmune-prone BAFF transgenic mice lacking marginal zone B cells. Eur. J. Immunol. 36, 2504–2514 (2006).
Nguyen, C. Q., Kim, H., Cornelius, J. G. & Peck, A. B. Development of Sjögren's syndrome in nonobese diabetic-derived autoimmune-prone C57BL/6. NOD-Aec1Aec2 mice is dependent on complement component-3. J. Immunol. 179, 2318–2329 (2007).
Shen, L. et al. Development of autoimmunity in IL-14α-transgenic mice. J. Immunol. 177, 5676–5686 (2006).
Shen, L. et al. Central role for marginal zone B cells in an animal model of Sjögren's syndrome. Clin. Immunol. 168, 30–36 (2016).
Daridon, C. et al. Identification of transitional type II B cells in the salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 54, 2280–2288 (2006).
Nocturne, G. & Mariette, X. Sjögren syndrome-associated lymphomas: an update on pathogenesis and management. Br. J. Haematol. 168, 317–327 (2015).
Mauri, C. & Blair, P. A. Regulatory B cells in autoimmunity: developments and controversies. Nat. Rev. Rheumatol. 6, 636–643 (2010).
Blair, P. A. et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 (2010).
Lemoine, S., Morva, A., Youinou, P. & Jamin, C. Human T cells induce their own regulation through activation of B cells. J. Autoimmun. 36, 228–238 (2011).
Candando, K. M., Lykken, J. M. & Tedder, T. F. B10 cell regulation of health and disease. Immunol. Rev. 259, 259–272 (2014).
Menon, M., Blair, P. A., Isenberg, D. A. & Mauri, C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 44, 683–697 (2016).
Wang, R.-X. et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641 (2014).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014).
Fogel, O. et al. Role of the IL-12/IL-35 balance in patients with Sjögren syndrome. J. Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2017.07.041 (2017).
Puga, I. et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180 (2011).
Magri, G. et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 15, 354–364 (2014).
Davies, R. et al. Patients with primary Sjögren's syndrome have alterations in absolute quantities of specific peripheral leucocyte populations. Scand. J. Immunol. 86, 491–502 (2017).
Mackay, F. et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710 (1999).
Mariette, X. et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann. Rheum. Dis. 62, 168–171 (2003).
Lavie, F. et al. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren's syndrome. J. Pathol. 202, 496–502 (2004).
Daridon, C. et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 56, 1134–1144 (2007).
Lavie, F. et al. B-Cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjögren's syndrome. Scand. J. Immunol. 67, 185–192 (2008).
Lahiri, A. et al. Specific forms of BAFF favor BAFF receptor-mediated epithelial cell survival. J. Autoimmun. 51, 30–37 (2014).
Nardelli, B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001).
Litinskiy, M. B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Sjöstrand, M. et al. The expression of BAFF is controlled by IRF transcription factors. J. Immunol. 196, 91–96 (2016).
Brkic, Z. et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren's syndrome and association with disease activity and BAFF gene expression. Ann. Rheum. Dis. 72, 728–735 (2013).
Ittah, M. et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur. J. Immunol. 38, 1058–1064 (2008).
Ittah, M. et al. Induction of B cell-activating factor by viral infection is a general phenomenon, but the types of viruses and mechanisms depend on cell type. J. Innate Immun. 3, 200–207 (2011).
Groom, J. et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J. Clin. Invest. 109, 59–68 (2002).
Ding, J. et al. BAFF overexpression increases lymphocytic infiltration in Sjögren's target tissue, but only inefficiently promotes ectopic B-cell differentiation. Clin. Immunol. 169, 69–79 (2016).
Bombardieri, M. et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J. Immunol. 189, 3767–3776 (2012).
Salomonsson, S. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 48, 3187–3201 (2003).
Le Pottier, L. et al. Ectopic germinal centers are rare in Sjögren's syndrome salivary glands and do not exclude autoreactive B cells. J. Immunol. 182, 3540–3547 (2009).
Bombardieri, M., Lewis, M. & Pitzalis, C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat. Rev. Rheumatol. 13, 141–154 (2017).
Förster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Luther, S. A., Lopez, T., Bai, W., Hanahan, D. & Cyster, J. G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481 (2000).
Nocturne, G. et al. CXCL13 and CCL11 serum levels and lymphoma and disease activity in primary Sjögren's syndrome. Arthritis Rheumatol. 67, 3226–3233 (2015).
Barone, F. et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjögren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J. Immunol. 180, 5130–5140 (2008).
Barone, F. et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc. Natl Acad. Sci. USA 112, 11024–11029 (2015).
Ciccia, F. et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjögren's syndrome. Ann. Rheum. Dis. 71, 295–301 (2012).
Cella, M., Otero, K. & Colonna, M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1ß reveals intrinsic functional plasticity. Proc. Natl Acad. Sci. USA 107, 10961–10966 (2010).
Ciccia, F. et al. Interleukin (IL)-22 receptor 1 is over-expressed in primary Sjögren's syndrome and Sjögren-associated non-Hodgkin lymphomas and is regulated by IL-18. Clin. Exp. Immunol. 181, 219–229 (2015).
Kang, K. Y. et al. Impact of interleukin-21 in the pathogenesis of primary Sjögren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res. Ther. 13, R179 (2011).
Maehara, T. et al. Selective localization of T helper subsets in labial salivary glands from primary Sjögren's syndrome patients. Clin. Exp. Immunol. 169, 89–99 (2012).
Gong, Y.-Z. et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren's syndrome. J. Autoimmun. 51, 57–66 (2014).
Blokland, S. L. M. et al. Elevated CCL25 and CCR9-expressing T helper cells in salivary glands of primary Sjögren's syndrome patients: potential new axis in lymphoid neogenesis. Arthritis Rheumatol. 69, 2038–2051 (2017).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Verstappen, G. M. et al. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren's syndrome. Arthritis Rheumatol. 69, 1850–1861 (2017).
Gommerman, J. L., Rojas, O. L. & Fritz, J. H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 5, 652–662 (2014).
Szyszko, E. A. et al. Salivary glands of primary Sjögren's syndrome patients express factors vital for plasma cell survival. Arthritis Res. Ther. 13, R2 (2011).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 76, 9–16 (2017).
Clancy, R. M. et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J. Clin. Invest. 116, 2413–2422 (2006).
Shen, L. et al. Novel autoantibodies in Sjögren's syndrome. Clin. Immunol. 145, 251–255 (2012).
Baer, A. N., Petri, M., Sohn, J., Rosen, A. & Casciola-Rosen, L. Association of antibodies to interferon-inducible protein-16 with markers of more severe disease in primary Sjögren's syndrome. Arthritis Care Res. 68, 254–260 (2016).
Leadbetter, E. A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Lindop, R. et al. Molecular signature of a public clonotypic autoantibody in primary Sjögren's syndrome: a 'forbidden' clone in systemic autoimmunity. Arthritis Rheum. 63, 3477–3486 (2011).
Thurgood, L. A. et al. An immunodominant La/SSB autoantibody proteome derives from public clonotypes. Clin. Exp. Immunol. 174, 237–244 (2013).
Theander, E. et al. Lymphoma and other malignancies in primary Sjögren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann. Rheum. Dis. 65, 796–803 (2006).
Smedby, K. E. et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J. Natl Cancer Inst. 98, 51–60 (2006).
Weng, M.-Y., Huang, Y.-T., Liu, M.-F. & Lu, T.-H. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjögren's syndrome in Taiwan. Ann. Rheum. Dis. 71, 524–527 (2012).
Johnsen, S. J. et al. Risk of non-Hodgkin's lymphoma in primary Sjögren's syndrome: a population-based study. Arthritis Care Res. 65, 816–821 (2013).
Martin, T. et al. Salivary gland lymphomas in patients with Sjögren's syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 43, 908–916 (2000).
Bende, R. J. et al. Among B cell non-Hodgkin's lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 201, 1229–1241 (2005).
Song, H., Tong, D., Cha, Z. & Bai, J. C-X-C chemokine receptor type 5 gene polymorphisms are associated with non-Hodgkin lymphoma. Mol. Biol. Rep. 39, 8629–8635 (2012).
Theander, E. et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann. Rheum. Dis. 70, 1363–1368 (2011).
Risselada, A. P. et al. The prognostic value of routinely performed minor salivary gland assessments in primary Sjögren's syndrome. Ann. Rheum. Dis. 73, 1537–1540 (2014).
Haacke, E. A. et al. Germinal centres in diagnostic labial gland biopsies of patients with primary Sjögren's syndrome are not predictive for parotid MALT lymphoma development. Ann. Rheum. Dis. 76, 1781–1784 (2017).
Quartuccio, L. et al. BLyS upregulation in Sjogren's syndrome associated with lymphoproliferative disorders, higher ESSDAI score and B-cell clonal expansion in the salivary glands. Rheumatology 52, 276–281 (2013).
Steri, M. et al. Overexpression of the cytokine BAFF and autoimmunity risk. N. Engl. J. Med. 376, 1615–1626 (2017).
Gottenberg, J.-E. et al. No evidence for an association between the -871 T/C promoter polymorphism in the B-cell-activating factor gene and primary Sjögren's syndrome. Arthritis Res. Ther. 8, R30 (2006).
Nezos, A. et al. B-Cell activating factor genetic variants in lymphomagenesis associated with primary Sjögren's syndrome. J. Autoimmun. 51, 89–98 (2014).
Novak, A. J. et al. Genetic variation in B-cell-activating factor is associated with an increased risk of developing B-cell non-Hodgkin lymphoma. Cancer Res. 69, 4217–4224 (2009).
Papageorgiou, A. et al. A BAFF receptor His159Tyr mutation in Sjögren's syndrome-related lymphoproliferation. Arthritis Rheumatol. 67, 2732–2741 (2015).
Tobón, G. J. et al. The Fms-like tyrosine kinase 3 ligand, a mediator of B cell survival, is also a marker of lymphoma in primary Sjögren's syndrome. Arthritis Rheum. 62, 3447–3456 (2010).
Sisto, M. et al. A failure of TNFAIP3 negative regulation maintains sustained NF-κB activation in Sjögren's syndrome. Histochem. Cell Biol. 135, 615–625 (2011).
Johnsen, S. J. et al. Low protein A20 in minor salivary glands is associated with lymphoma in primary Sjögren's syndrome. Scand. J. Immunol. 83, 181–187 (2016).
Musone, S. L. et al. Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes Immun. 12, 176–182 (2011).
Dass, S. et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 67, 1541–1544 (2008).
Meijer, J. M. et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 960–968 (2010).
Devauchelle-Pensec, V. et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann. Intern. Med. 160, 233–242 (2014).
Bowman, S. J. et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren's syndrome. Arthritis Rheumatol. 69, 1440–1450 (2017).
Bootsma, H., Kroese, F. G. M. & Vissink, A. Rituximab in the treatment of Sjögren's syndrome: Is it the right or wrong drug? Arthritis Rheumatol. 69, 1346–1349 (2017).
Gottenberg, J.-E. et al. Efficacy of rituximab in systemic manifestations of primary Sjögren's syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann. Rheum. Dis. 72, 1026–1031 (2013).
Mariette, X. et al. Efficacy and safety of belimumab in primary Sjögren's syndrome: results of the BELISS open-label phase II study. Ann. Rheum. Dis. 74, 526–531 (2015).
Seror, R. et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjögren's syndrome. Ann. Rheum. Dis. 66, 351–357 (2007).
Pollard, R. P. E. et al. Serum levels of BAFF, but not APRIL, are increased after rituximab treatment in patients with primary Sjögren's syndrome: data from a placebo-controlled clinical trial. Ann. Rheum. Dis. 72, 146–148 (2013).
Lavie, F. et al. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann. Rheum. Dis. 66, 700–703 (2007).
Cornec, D. et al. Blood and salivary-gland BAFF-driven B-cell hyperactivity is associated to rituximab inefficacy in primary Sjögren's syndrome. J. Autoimmun. 67, 102–110 (2016).
Pers, J.-O. et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren's syndrome. Arthritis Rheum. 56, 1464–1477 (2007).
Merrill, J. T. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a 24-week randomized, placebo-controlled, phase IIb study. Arthritis Rheumatol. https://doi.org/10.1002/art.40360 (2017).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Lokhorst, H. M. et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 373, 1207–1219 (2015).
Mauri, C. & Menon, M. The expanding family of regulatory B cells. Int. Immunol. 27, 479–486 (2015).
Kalampokis, I., Yoshizaki, A. & Tedder, T. F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 15 (Suppl. 1), S1 (2013).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02291029 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02334306 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01782235 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02149420 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02610543 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02631538 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02067910 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02915159 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02780674 (2017).
Acknowledgements
The work of the authors was supported financially by the Fondation Pour La Recherche Médicale DEQ20150934719: Sjögren's Syndrome and Autoimmunity-Associated Lymphomas (SAIL).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nocturne, G., Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat Rev Rheumatol 14, 133–145 (2018). https://doi.org/10.1038/nrrheum.2018.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.1
This article is cited by
-
Manifestations and management of Sjögren’s disease
Arthritis Research & Therapy (2024)
-
Increased METTL3 expression and m6A RNA methylation may contribute to the development of dry eye in primary Sjögren’s syndrome
BMC Ophthalmology (2023)
-
Anti-SSA/SSB-negative primary Sjögren’s syndrome showing different clinical phenotypes: a retrospective study of 934 cases
Advances in Rheumatology (2023)
-
Iguratimod suppresses Tfh cell differentiation in primary Sjögren’s syndrome patients through inhibiting Akt/mTOR/STAT3 signaling
Arthritis Research & Therapy (2023)
-
Sex differences in the percentage of IRF5 positive B cells are associated with higher production of TNF-α in women in response to TLR9 in humans
Biology of Sex Differences (2023)